MINING.COM Staff Writer | October 12, 2021
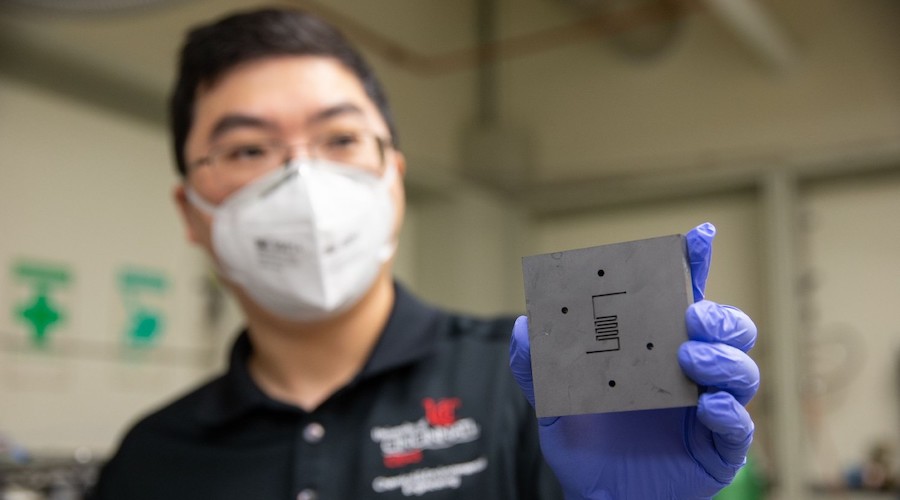
UC chemical engineer Jingjie Wu holds up the reactor where a catalyst converts carbon dioxide into methane. (Image by Andrew Higley, courtesy of UC Creative + Brand).
Astronauts roaming Mars could save half the fuel for a return trip home by making what they need on the red planet once they arrive.

According to engineers at the University of Cincinnati, space explorers could follow the updated version of an old process that involves using a carbon catalyst in a reactor to convert carbon dioxide into methane.
In detail, the UofC researchers have developed a process based on the “Sabatier reaction,” which is a process the International Space Station uses to scrub the carbon dioxide from the air the astronauts breathe and generate rocket fuel to keep the station in high orbit.
But since the Martian atmosphere is composed almost entirely of carbon dioxide, astronauts could treat it like a gas station and easily pump carbon dioxide through the reactor and produce methane for a rocket.
In a paper published in the journal Nature Communications, the experts explain that the process of capturing and transforming CO2 also holds promise to help mitigate climate change, while producing fuel on Earth as a byproduct.
“The Biden Administration has set a goal of achieving a 50% reduction in greenhouse gas pollutants by 2030 and an economy that relies on renewable energy by 2050,” senior researcher Jingjie Wu said in a media statement. “That means we’ll have to recycle carbon dioxide.”
Wu and his students, including lead author Tianyu Zhang, are also experimenting with different catalysts such as graphene quantum dots — layers of carbon just nanometers big — that can increase the yield of methane.
“The process is 100 times more productive than it was just 10 years ago. So you can imagine that progress will come faster and faster,” Wu said. “In the next 10 years, we’ll have a lot of startup companies to commercialize this technique.”
Wu pointed out that his students are also applying this technique with different catalysts to produce ethylene. Called the world’s most important chemical, ethylene is used in the manufacture of plastics, rubber, synthetic clothing and other products.
No comments:
Post a Comment