Largest decentralized study of its kind shows high levels of engagement with study app
A new study, which was a collaboration between Huma Therapeutics and the Medical Research Council Epidemiology Unit at the University of Cambridge, shows that participants using Huma's clinical trial platform had high, sustained levels of engagement in an observational, fully remote COVID-19 study.
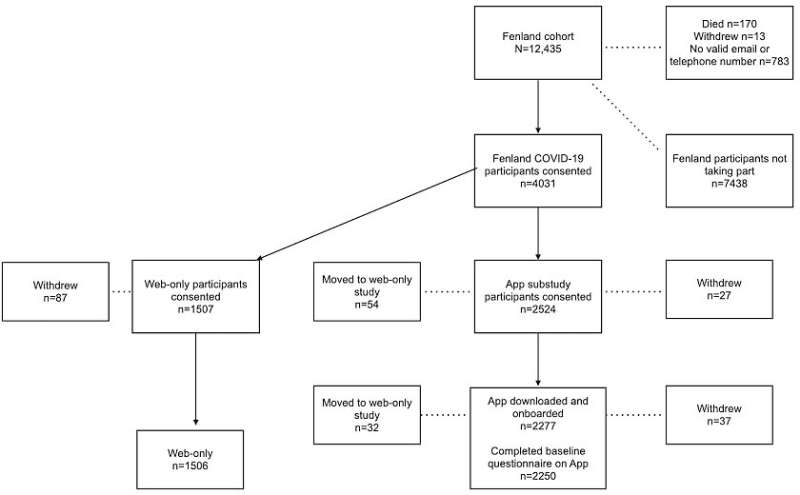
The study, published in the Journal of Medical Internet Research, involved 62.61% (2524/4031) participants from the longitudinal Fenland study, making it the largest population-based study to-date exploring how digital technologies can support population research.
From the participants, 90.21% (2277/2524) completed the app-based onboarding process and signed e-consent. In addition to using the study app (available for both iPhone and Android), each was also sent a digital pulse oximeter (to measure blood oxygen levels) and thermometer. They were provided with remote set-up assistance and were asked to record the following biomarkers:
- blood oxygen saturation (three times per week)
- body temperature (three times per week)
- resting heart rate (three times per week)
- activity levels (measured passively)
- respond to monthly questionnaires
- blood spot samples to test for the presence of coronavirus antibodies.
Participants had a positive experience with the study app, finding it easy to use and quick to report measurements and symptoms. They took part in the observational study for at least 6 months and most kept completing measures until asked to stop; there was minimum drop off in engagement over the study period. On average, people used the study app for 34.5 weeks (7.9 months), with only 2.5% of participants withdrawing from the study. It was interesting to see a higher engagement rate amongst the participants aged over 65.
Dr. Arrash Yassaee, Global Clinical Director at Huma, said, "Huma is committed to building robust clinical and scientific evidence for its technology. The high level of engagement and retention we've seen in this study is very encouraging. User-friendly clinical trial technologies such as Huma's have great potential to transform population-based health research by increasing access and reducing the burden on participants. This kind of data collection is incredibly valuable for understanding health and disease processes in the real world and gathering insights that can make a difference to people's lives."
Dr. Kirsten Rennie, a Senior Research Associate at MRC Epidemiology Unit and an expert in quantitative measures of physical activity and diet, who led the study said, "Enrolment and retention in traditional cohort-based observational studies is a constant challenge and participation has been declining in recent years. Here we saw not only great enrolment and retention, but also engagement which has helped us create a useful checklist for other researchers to follow."
The COVID-19 pandemic accelerated interest in the use of digital health solutions for remote health monitoring. But while these technologies have shown benefits for patients with chronic or acute health conditions, less is known about their utility in population-based health research, where it is becoming increasingly hard to recruit participants and keep them engaged over months or even years.
More information: Kirsten L Rennie et al, Engagement With mHealth COVID-19 Digital Biomarker Measurements in a Longitudinal Cohort Study: Mixed Methods Evaluation, Journal of Medical Internet Research (2022). DOI: 10.2196/40602
No comments:
Post a Comment