Melting glasses: The glass-to-liquid transition
The process of liquid-to-glass transition is a complex procedure in science, as is the glass-to-liquid transition known as glass melting. In a new report published in Science Advances, Qi Zhang and a research team in physics at the Hong Kong University of Science and Technology in China assembled colloidal glasses via vapor deposition and melted them to observe the glass transition dynamics.
The structural and dynamic parameters saturated at different depths to define a surface liquid layer and a glassy intermediate layer. The scientists observed single-particle kinetics with various features to confirm the theoretical predictions of melting for the glass surface layer.
The dynamics of melting glass
The process of glass melting is not, as assumed, a reverse process of the glass-forming transition from liquid-to-glass. The mechanism of glass melting is at the preliminary stage of development, in contrast to the intensively studied mechanism of glass forming transitions. Ultrastable glasses have shown heterogenous surface melting in a mechanism of surface pre-melting to preempt melting from within.
Polymer scientists had studied atomic and molecular ultrastable glasses, and described colloids as outstanding model systems to study glass-melting behavior due to micron-scale particles and thermal motions that can be viewed via optical microscopy. Colloids provide important microscopic information on bulk glasses including insights to shear-induced bulk glass melting.
Researchers have yet to explore thermally induced bulk or surface melting at the single particle level since it requires colloids with tunable attraction. In this work, Zhang and colleagues used attractive colloids to measure microscopic kinetics at diverse temperature ranges, to examine slow and fast temperature changes for monolayer and multilayer samples, and understand their pre-melting and melting trajectories.
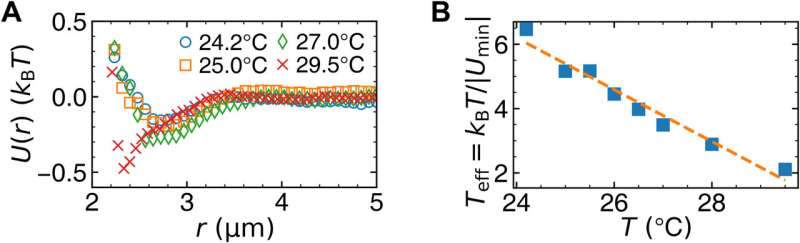
![Surface premelting under slow temperature change. At 27.0° (A and B) and 25.4°C (C and D), particles in the monolayer are colored by ρ and log(DW), respectively. Scale bars, 20 μm. (E) At 26.0°C, the profiles of the structural parameters { ρ~(y) and s~2(y) } fitted by Eq. 3 (red solid curve); the profiles of the dynamic parameters { log[τ(y)]~ , log[ph(y)]~ , and log[DW(y)]~ } fitted by Eq. 4 (black dashed curve). The five regimes (vapor, dense vapor, liquid, glassy layer, and bulk glass) have four interfaces labeled on the top x axis; their positions ys,0,1,2 are defined at ρ~=5,50,and95% and log(DW)~=95% , respectively. y = 0 is defined as y0 at 27.0°C [yellow dashed lines in (A) and (B)]. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf1101 Melting glasses – the glass-to-liquid transition](https://scx1.b-cdn.net/csz/news/800a/2023/melting-glasses--the-g-1.jpg)
The glass-melting experiments: Surface pre-melting under slow temperature changes
During the experiments, Zhang and the team incorporated a 50:50 mixture of polymer spheres to overcome crystallization and added a dye to induce attraction between polymethyl methacrylate spheres. They pumped the dye to the unheated region via thermophoresis to decrease the attraction strength, while linearly increasing the effective temperature.
The outcomes yielded monolayer and multilayer colloids. The team assembled the colloidal glasses via vapor deposition to form ultrastable molecular glasses. They noted the particles via optical microscopy and tracked particulate Brownian motions with image analysis.
The impact of slow temperature change on the structural and dynamic parameters
The scientists noted complete bulk melting transitions at 25.3 degrees Celsius. During the process of crystal pre-melting, researchers had predicted the power-law growth of the surface liquid thickness in theory, and observed the outcomes experimentally, and with simulations. The team quantified the relationship between the local structure and dynamics, where the low-density region near the surface exhibited mode-coupling transition behavior of fragile glass while the high-density region near the bulk exhibited the Arrhenius behavior of strong glass.
This fragile-to-strong crossover with decreasing temperature was also seen in water, metallic glasses, and organic/inorganic glasses. The present research focus on the structural dynamic correlation of bulk glass and supercooled liquid provided a connection near the surface.
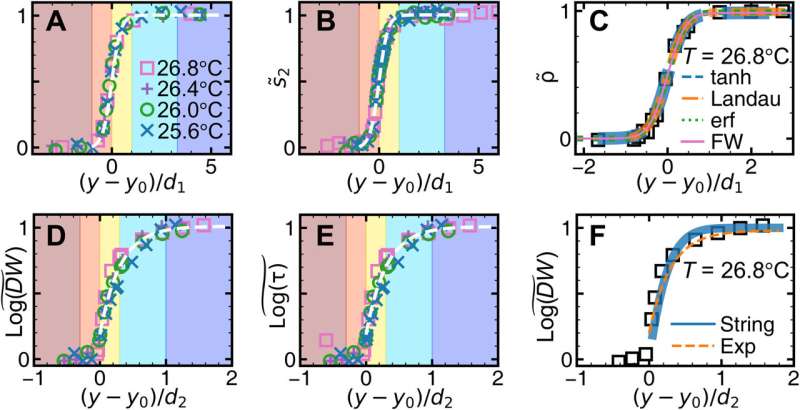
![Surface melting under the fast temperature change. (A to C) The monolayer sample colored by log(DW) at different times. Scale bars, 20 μm. (D to F) ρ(y) and log[DW(y)] of (A) to (C) fitted by Eqs. 3 (solid curves) as well as 4 (dashed curves) and 5 (dotted curves), respectively. They share the same double y axes. The colored regions are labeled in (H). (G) Density profiles across the glassy layer at different times fitted with Eq. 3 (solid curves). (H) Evolution of the surface layers. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf1101 Melting glasses – the glass-to-liquid transition](https://scx1.b-cdn.net/csz/news/800a/2023/melting-glasses--the-g-3.jpg)
Multilayer dynamics and temperature changes
While the monolayer and bilayer colloidal crystals showed distinctly different surface pre-melting and melting behaviors, the monolayer and multilayer colloidal glasses maintained similarities during melting and pre-melting. Crystal melting is typically observed by abruptly increasing the temperature above the melting point. To facilitate this, the team abruptly changed the temperature mode to study both melting and pre-melting processes in glass.
The glass transition temperature was lower under fast temperature changes, when compared to the value at slow temperature changes. The bilayer and trilayer glasses subjected to fast temperature change exhibited similar pre-melting behaviors. The researchers had already observed the consistent speed of melting in ultrastable glass without an experimental test and with simulations, which agreed with the observations made in this study.
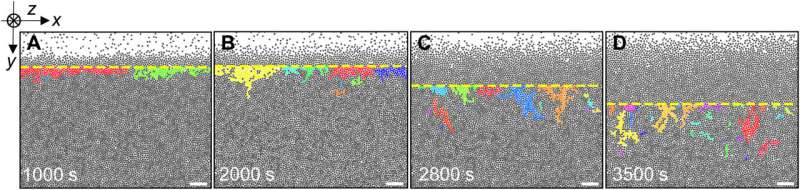
Cooperative rearrangement regions
Zhang and the team noted cooperative rearrangement regions that are crucial to glass relaxation near the surface. They defined these regions as clusters composed of at least two mobile particles and assumed them to contain a compact core surrounded by a string-like shell.
As the effective temperature increased with time, the morphology of the material changed from compact to string-like compositions, as predicted and observed in bulk glasses. During the process of heating, the polarized cooperative rearrangement region on the surface of the monolayer glass changed from parallel to nearly perpendicular, to facilitate melting. The reverse was true for these regions during glass growth via vapor deposition.
Outlook
In this way, Qi Zhang and colleagues conducted single-particle kinetics to reveal two surface layers in glass. The liquid layer on the top remained stable at a fixed temperature instead of propagating into the bulk to indicate pre-melting rather than melting behavior. They noted similarities between glass and crystals during the process of pre-melting and melting, for instance, ordinary glasses exhibited nucleation-like bulk melting, much like crystals to support the thermodynamic origin of glass transition. Polymer scientists are still at the preliminary stage of studying glass surface melting, which requires theoretical and experimental detail at the single-particle level.
Simulations had thus far focused on the melting front speed and crossover depth from surface to bulk melting, while the concept of glass pre-melting remains to be discussed in depth. The glass surface also showed an additional glassy layer in contrast to the crystal pre-melting processes—that go beyond the pre-melting theory. While the outcomes of pre-melting/melting behaviors observed here are similar to bulk glasses, they are in contrast to the behavioral dynamics of monolayer/bilayer crystals.
More information: Qi Zhang et al, Surface premelting and melting of colloidal glasses, Science Advances (2023). DOI: 10.1126/sciadv.adf1101
Hajime Tanaka et al, Revealing key structural features hidden in liquids and glasses, Nature Reviews Physics (2019). DOI: 10.1038/s42254-019-0053-3
Journal information: Science Advances
© 2023 Science X Network
Researchers prepare novel low-melting, nitrogen-containing, stannous chlorophosphate glass
No comments:
Post a Comment