WORST WEEK EVER A DIGEST
AstraZeneca to reduce EU Covid vaccine deliveries following production problems
Issued on: 15/03/2021 -

Text by: NEWS WIRES
The European Union is facing further shortfalls in its coronavirus inoculation programme after pharmaceutical giant AstraZeneca said production problems and export restrictions would reduce planned deliveries of its vaccine.
The Anglo/Swedish firm's image has already taken a hit with several countries suspending the rollout of its vaccine over blood clot fears, even as the World Health Organization said there was no reason to stop using it.
It is just the latest blow for the AstraZeneca vaccine, which is the cheapest vaccine aimed at fighting back against a pandemic that has claimed more than 2.6 million lives worldwide.
Germany has already reported adverse effects due to the delay, the state of Thuringia cancelling appointments and suspending a pilot project for general practitioners to administer the vaccine.
>> How the EU’s Covid-19 vaccine rollout became an ‘advert for Brexit’
The head of the country's disease control agency Robert Koch Institute, Lothar Wieler, meanwhile warned that "the third wave has already started in Germany".
Despite the worrying signs, thousands joined protests in German cities on Saturday against anti-Covid measures.
France on a 'razor's edge', says PM
French Prime Minister Jean Castex said his government still expected to exceed its target of 10 million vaccinated by April 15, though he said some labs were not respecting delivery deadlines.
Castex defended the use of the AstraZeneca vaccine despite precautions taken by other nations.
>> New strain of Covid-19 tripled infections despite UK lockdown
"I would not allow myself to send poison to my fellow citizens," he said during a visit to a vaccination centre.
He also did not rule out a new lockdown in the Ile-de-France region, which is home to the capital Paris, saying he was ready to take "additional measures" if necessary.
"We are on a razor's edge," he told Le Monde newspaper, as the first three intensive care patients were moved from Ile-de-France to nearby regions on Saturday to relieve the pressure on overwhelmed hospitals in the capital.
Hospital in Jordan runs out of oxygen
The United States, the country hit hardest by the pandemic, has ramped up its vaccination programme after a shaky start.
The Centers for Disease Control and Prevention said 100 million doses had been administered so far, just less than a third of the total given worldwide so far.
Tunisia and Ethiopia both launched vaccination campaigns on Saturday, with Ethiopian officials flagging an alarming rise in cases.
There was outrage in Jordan after at least seven Covid-19 patients died on Saturday when a hospital ran out of oxygen.
"I have submitted my resignation to the prime minister," said health minister Nazir Obeidat, adding that he took "full moral responsibility" for what happened.
WHO: No link between AstraZeneca jab, blood clotting
Several countries suspended the use of AstraZeneca's vaccine this week, with Norway reporting an "unexpected death from a brain haemorrhage" after receiving the shot.
Norwegian officials added on Saturday that the country had "received several adverse event reports about younger vaccinated people with bleeding under the skin" after getting the shot.
It also said it had received "three more reports of severe cases of blood clots or brain haemorrhages in younger people who have received the AstraZeneca vaccine".
The World Health Organization, which said its vaccines advisory committee was examining the safety data coming in, has stressed that no causal link has been established between the AstraZeneca vaccine and blood clotting.
"Yes, we should continue using the AstraZeneca vaccine," WHO spokeswoman Margaret Harris said Friday, stressing that any concerns over safety must be investigated.
AstraZeneca insisted its jab was safe, adding there was "no evidence" of higher blood clot risks.
Italy and Austria have banned the use of jabs from separate batches of AstraZeneca, and Thailand and Bulgaria said this week they would delay their rollout.
Austria, the Czech Republic, Slovenia, Bulgaria and Latvia meanwhile called for EU talks to discuss "huge disparities" in vaccine distribution, according to a letter published on Saturday.
"If this system were to carry on, it would continue creating and exacerbating huge disparities among member states by this summer, whereby some would be able to reach herd immunity in a few weeks while others would lag far behind," the letter said.
Italy on Friday announced tough new restrictions, with schools, restaurants, shops and museums ordered to close across most regions of Italy, including Rome and Milan from next week.
(AFP)
By Thomas Escritt, Stephanie Nebehay
BERLIN/GENEVA (Reuters) - Germany, France and Italy said on Monday they would suspend AstraZeneca COVID-19 shots after several countries reported possible serious side-effects, but the World Health Organization

(WHO) said there was no proven link and people should not panic.
Still, the decision by the European Union’s three biggest countries to put inoculations with the AstraZeneca shot on hold threw the already struggling vaccination campaign in the 27-nation EU into disarray.
Denmark and Norway stopped giving the shot last week after reporting isolated cases of bleeding, blood clots and a low platelet count. Iceland and Bulgaria followed suit and Ireland and the Netherlands announced suspensions on Sunday.
Spain will stop using the vaccine for at least 15 days, Cadena Ser radio reported, citing unnamed sources.
The top WHO scientist reiterated on Monday that there have been no documented deaths linked to COVID-19 vaccines.
“We do not want people to panic,” Soumya Swaminathan said on a virtual media briefing, adding there has been no association, so far, pinpointed between so-called “thromboembolic events” reported in some countries and COVID-19 shots.
The moves by some of Europe’s largest and most populous countries will deepen concerns about the slow rollout of vaccines in the region, which has been plagued by shortages due to problems producing vaccines, including AstraZeneca’s.
Germany warned last week it was facing a third wave of infections, Italy is intensifying lockdowns and hospitals in the Paris region are close to being overloaded.
German Health Minister Jens Spahn said that although the risk of blood clots was low, it could not be ruled out.
“This is a professional decision, not a political one,” Spahn said, adding he was following a recommendation of the Paul Ehrlich Institute, Germany’s vaccine regulator.
France said it was suspending the vaccine’s use pending an assessment by the EU medicine regulator due on Tuesday. Italy said its halt was a “precautionary and temporary measure” pending the regulator’s ruling.
Austria and Spain have stopped using particular batches and prosecutors in the northern Italian region of Piedmont earlier seized 393,600 doses following the death of a man hours after he was vaccinated. It was the second region to do so after Sicily, where two people had died shortly after having their shots.
The WHO appealed to countries not to suspend vaccinations against a disease that has caused more than 2.7 million deaths worldwide. WHO chief Tedros Adhanom Ghebreyesus said systems were in place to protect public health.
“This does not necessarily mean these events are linked to COVID-19 vaccination, but it’s routine practice to investigate them, and it shows that the surveillance system works and that effective controls are in place,” Tedros said during a virtual media briefing in Geneva.
RELATED COVERAGE
EU regulator to meet on Thursday to discuss AstraZeneca vaccine
He said an advisory committee meeting on AstraZeneca would be held on Tuesday.
The United Kingdom said it had no concerns, while Poland said it thought the benefits outweighed any risks.
The EMA has said that as of March 10, a total of 30 cases of blood clotting had been reported among close to 5 million people vaccinated with the AstraZeneca shot in the European Economic Area, which links 30 European countries.
Michael Head, a senior research fellow in global health at the University of Southampton, said the decisions by France, Germany and others looked baffling.
“The data we have suggests that numbers of adverse events related to blood clots are the same (and possibly, in fact lower) in vaccinated groups compared to unvaccinated populations,” he said, adding that halting a vaccination programme had consequences.
“This results in delays in protecting people, and the potential for increased vaccine hesitancy, as a result of people who have seen the headlines and understandably become concerned. There are no signs yet of any data that really justify these decisions.”
Italian medicine agency Aifa’s general director, Nicola Magrini, told a radio station that several European countries preferred to suspend the vaccine “in the presence of some very recent and very few cases of adverse events” in women and young people.
“...Those who have already had the vaccine can and must remain safe,” she said. “I feel like saying the vaccine is safe, even having reviewed all the data.”
‘UNUSUAL’ SYMPTOMS
AstraZeneca’s shot was among the first and cheapest to be developed and launched at volume since the coronavirus was first identified in central China at the end of 2019, and is set to be the mainstay of vaccination programmes in much of the developing world.
Thailand announced plans on Monday to go ahead with the Anglo-Swedish firm’s shot after suspending its use on Friday, but Indonesia said it would wait for the WHO to report.
The WHO said its advisory panel was reviewing reports related to the shot and would release its findings as soon as possible. But it said it was unlikely to change its recommendations, issued last month, for widespread use, including in countries where the South African variant of the virus may reduce its efficacy.
The EMA has also said there was no indication the events were caused by the vaccination and that the number of reported blood clots was no higher than seen in the general population.
The handful of reported side-effects in Europe have upset vaccination programmes already stumbling over slow rollouts and vaccine scepticism in some countries.
The Netherlands said on Monday it had seen 10 cases of possible noteworthy adverse side-effects from the AstraZeneca shot, hours after putting its vaccination programme on hold following reports of potential side-effects in other countries.
Denmark reported “highly unusual” symptoms in a 60-year-old citizen who died from a blood clot after receiving the vaccine, the same phrase used on Saturday by Norway about three people under the age of 50 it said were being treated in hospital.
“It was an unusual course of illness around the death that made the Danish Medicines Agency react,” the agency said in a statement late on Sunday.
One of the three health workers hospitalised in Norway after receiving the AstraZeneca shot had died, health authorities said on Monday, but there was no evidence the vaccine was the cause.
AstraZeneca said earlier it had conducted a review covering more than 17 million people vaccinated in the European Union and the UK which had shown no evidence of an increased risk of blood clots.
Long-awaited results from AstraZeneca 30,000-person U.S. vaccine trial are currently being reviewed by independent monitors to determine whether the shot is safe and effective, a top U.S. official said on Monday.
Reporting by Panarat Thepgumpanat in BANKOK and Andreas Rinke and Paul Carrel in BERLIN, Angelo Amante in ROME, Christian Lowe in PARIS, Toby Sterling in AMSTERDAM, Jacob Gronholt-Pedersen in COPENHAGEN, Kate Kelland in LONDON, Emilio Parodi in MILAN, Nathan Allen in MADRID, Emma Farge in GENEVA and Stanley Widianto in JAKARTA; writing by Philippa Fletcher; editing by Nick Macfie and Mark Heinrich
Coronavirus vaccine: WHO statement on Astra Zeneca jab safety
By Aidan Barlow @ArgusAidan
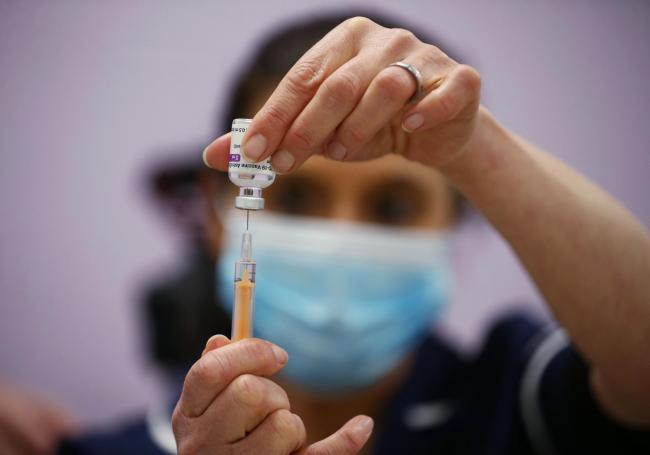
VACCINE LATEST
Suzie Shakespeare, Senior Immunisation Nurse, prepares a dose of Oxford/Astra Zeneca Covid-19 vaccine, during a mass vaccination of members of the public at Robertson House, Stevenage. Picture date: Tuesday February 9, 2021. PA Photo. Photo credit
COUNTRIES should continue to use the AstraZeneca Covid-19 vaccine, the World Health Organisation (WHO) says.
The WHO said there was no indication of a link between the jabs and blood clots.
It comes after Thailand said it will delay use of the AstraZeneca vaccine after several European countries temporarily suspended the jabs following a small number of reports of health problems.
Speaking via videoconference in Geneva, WHO director general Tedros Adhanom Ghebreyesus said: “As countries roll out Covid-19 vaccines, WHO is continuing to keep a close eye on their safety.
“WHO is aware that some countries have suspended the use of AstraZeneca vaccines based on reports of blood clots in some people who have received doses of the vaccine from two batches.
“This measure was taken as a precaution while a full investigation is finalised.
“It’s important to note that the European Medicines Agency has said there is no indication of a link between the vaccine and blood clots and that the vaccine can continue to be used while its investigation is ongoing.”
He said the findings and any change to the organisation’s recommendations following the investigation will be relayed to the public “immediately”.
His comments echoed earlier remarks made by WHO spokesperson Dr Margaret Harris, who described the vaccine as “excellent”.
Yong Poovorawan, an adviser to Thailand’s vaccination programme, said the delay, pending an investigation into the cause of reported side-effects, will not have a big impact on the rollout.
It came as AstraZeneca released a new statement saying there is no evidence of an increased risk of pulmonary embolism or deep vein thrombosis with the vaccine.
It said that, in fact, the occurrence is “significantly lower” in those who have been vaccinated than what would be expected among the general population.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has said there is no evidence to suggest the vaccine causes blood clot problems, and that people should still get their Covid-19 jab when asked to do so.
The European Medicines Agency (EMA) has also backed the jab’s safety and said there have been 30 reports of blood clots among close to five million people given the vaccine across Europe.
It said in a statement: “The position of EMA’s safety committee… is that the vaccine’s benefits continue to outweigh its risks and the vaccine can continue to be administered while investigation of cases of thromboembolic events is ongoing.”
On Thursday, Denmark, Norway and Iceland said they were temporarily halting all AstraZeneca vaccinations to investigate reports of blood clots among people who have had the jab.
Italy also followed Austria, Estonia, Latvia, Luxembourg and Lithuania in banning jabs from one particular batch of one million AstraZeneca vaccines, which was sent to 17 countries, after reports of a death.
AstraZeneca said in a statement on Friday: “An analysis of our safety data of more than 10 million records has shown no evidence of an increased risk of pulmonary embolism or deep vein thrombosis in any defined age group, gender, batch or in any particular country with Covid-19 vaccine AstraZeneca.
“In fact, the observed number of these types of events are significantly lower in those vaccinated than what would be expected among the general population.”
Earlier this week, the EMA reported that one person in Austria was diagnosed with blood clots and died 10 days after vaccination, but stressed there is “currently no indication that vaccination has caused these conditions”.
Another person was admitted to hospital with pulmonary embolism (blockage in arteries in the lungs) after being vaccinated.
Dr Phil Bryan, MHRA vaccines safety lead in the UK, said on Thursday: “Blood clots can occur naturally and are not uncommon.
“More than 11 million doses of the Covid-19 AstraZeneca vaccine have now been administered across the UK.
“Reports of blood clots received so far are not greater than the number that would have occurred naturally in the vaccinated population.”
Dr Bryan said the safety of the public always comes first and the issue is being kept under close review, “but available evidence does not confirm that the vaccine is the cause”.
Professor Anthony Harnden, deputy chairman of the Joint Committee on Vaccination and Immunisation, said: “Vaccine safety is critically important.
“Our UK regulator, the MHRA, reviews all reports of adverse events for both vaccines as they are reported.
“The public should have confidence that both vaccines used in the UK vaccination programme are safe and highly effective at preventing severe disease, including the prevention of blood clots caused by Covid.”
The European Medicines Agency and the World Health Organization say the data available do not suggest the vaccine caused the clots and that people should continue to be immunized
By Maria Cheng • Published 5 hours ago • Updated 3 hours ago
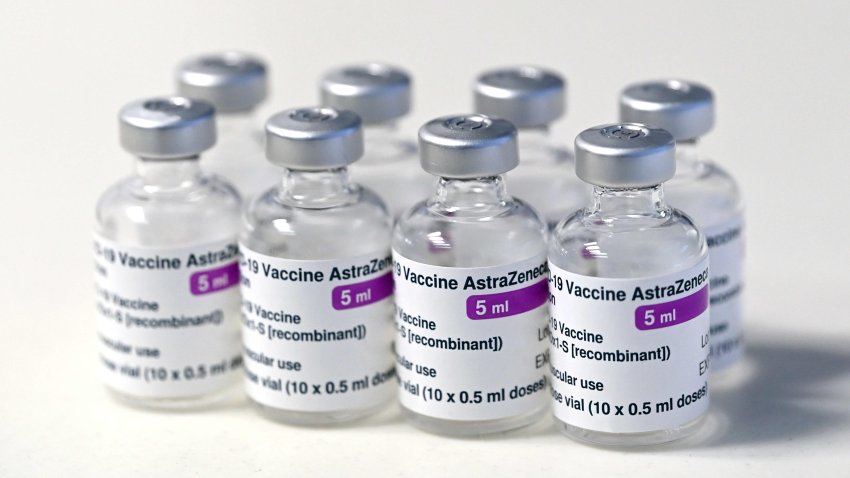
The Oxford-AstraZeneca covid vaccine.
In recent days, countries including Denmark, Ireland, Thailand, Germany, France and Italy have temporarily suspended their use of AstraZeneca's coronavirus vaccine after reports that some people who got a dose developed blood clots, even though there's no evidence that the shot was responsible.
The European Medicines Agency and the World Health Organization say the data available do not suggest the vaccine caused the clots and that people should continue to be immunized. Here's a look at we know — and what we don't.
WHAT HAPPENED?
Denmark was the first country to halt its use of the AstraZeneca COVID-19 vaccine last week after reports of blood clots in some people, including one person who developed multiple clots and died 10 days after receiving at least one dose. Danish health authorities said the suspension would last for at least two weeks while the cases were investigated, even as they noted that “at present, it cannot be concluded whether there is a link between the vaccine and the blood clots.”
Norway, Iceland, Bulgaria, Thailand, and Congo soon followed suit. On Saturday, Norwegian authorities reported that four people under age 50 who had gotten the AstraZeneca vaccine had an unusually low number of blood platelets. That could lead to severe bleeding. On Sunday, Ireland and the Netherlands announced that they, too, were stopping their use of the AstraZeneca vaccine temporarily.
On Monday, Germany, France and Italy also temporarily suspended use of the AstraZeneca vaccine.
Authorities in the Netherlands — like those elsewhere — said their suspension of the AstraZeneca vaccine was strictly precautionary.
“We must always err on the side of caution, which is why it is sensible to press the pause button now as a precaution,” said Hugo de Jonge, the Dutch health minister.
Still, several other countries have stuck with the vaccine.
In response to the suspensions of its vaccine, AstraZeneca said it had carefully reviewed the data on 17 million people who received doses across Europe. It said there was “no evidence of an increased risk” of blood clots in any age group or gender in any country.
IS THERE ANY PROOF THE VACCINE IS RESPONSIBLE?
No. The European Medicines Agency says there is “no indication that vaccination has caused these conditions." The EU regulator said the number of reports of blood clots in people who received the AstraZeneca vaccine was no higher than for those who hadn't gotten the shot.
In Britain, where 11 million doses of the AstraZeneca vaccine have been administered — more than any other country — there have been reports of about 11 people who developed blood clots after getting a shot. None were proven to have been caused by the vaccine.
Some doctors pointed out that since vaccination campaigns started by giving doses to the most vulnerable people, those now being immunized are more likely to already have health problems. Experts say that could make it difficult to determine whether a vaccine shot is responsible.
More Coronavirus News
SO WHY DID THEY STOP VACCINATION?
Any time vaccines are rolled out widely, scientists expect some serious health issues and deaths to be reported — simply because millions of people are receiving the shots and problems would be expected to occur randomly in a group so large. The vast majority of these end up not being connected to the vaccine, but because COVID-19 vaccines are still experimental, scientists must investigate every possibility that the shot could have some unforeseen side effects. The shots are considered experimental because the vaccines were only developed in the last year, so there is no long-term data for any of them.
“People die every day, and we have more than 300 million people globally who have been immunized who will die of other causes,” said Dr. Mariangela Simao, an assistant director-general at WHO.
IS THIS A CONCERN WITH OTHER COVID-19 VACCINES?
The EMA is currently examining whether COVID-19 shots made by Pfizer-BioNTech, Moderna Inc. and AstraZeneca might be causing low levels of blood platelets in some patients, a condition that could lead to bruising and bleeding.
4:04 Yes, It's Safe! Combatting COVID-19 Vaccine Hesitancy in Black and Brown CommunitiesHere’s Why Countries Are Halting the AstraZeneca Shot, Explained – NECNVaccine hesitancy in the Black and brown community is giving many of those in the most vulnerable populations pause before signing up for a COVID-19 vaccine. Physicians Jubril Oyeyemi and David Hayes-Bautista discuss how to combat vaccine fear in those communities.
HAS ASTRAZENECA RUN INTO OTHER TROUBLE?
The vaccine has been approved for use in adults in more than 50 countries and has been proven to be safe and effective in research done in Britain, Brazil and South Africa. But there have been concerns raised about how the vaccine data have been released, and some European leaders, including French President Emmanuel Macron, have questioned the vaccine's effectiveness.
Britain first authorized the vaccine based on partial results that suggested the shots were about 70% effective. But those results were clouded by a manufacturing mistake that led some participants to get just a half dose in their first shot — an error the researchers didn’t immediately acknowledge. When it recommended the vaccine be licensed, the EMA estimated the vaccine's efficacy to be about 60%.
The data on whether the vaccine protected older adults were also incomplete, leading some European countries to initially withhold the shot from older people.
In the U.S., the Food and Drug Administration suspended a study in 30,000 Americans for an unusual six weeks, as frustrated regulators sought information about some possible side effects reported in Britain.
“All the data we have seen about the AstraZeneca vaccine suggests it's very safe and is saving people from dying of COVID," said Dr. Paul Hunter, a professor of medicine at the University of East Anglia. “But this may be more of a perception problem because every time there is a vaccine issue, we hear the name ‘AstraZeneca’ soon after.”
SO WHAT ARE EXPERTS TELLING PEOPLE TO DO?
The WHO and the EMA — as well as regulators in several countries — say people should continue to be immunized and that the risk of getting vaccinated far outweighs any potential harm.
“The safety of the public will always come first," said Britain's drug regulator. “People should still go and get their COVID-19 vaccine when asked to do so.”
you are here: science media centre > roundups for journalists > expert reaction to reports that ireland has suspended the use of the oxford-astrazeneca vaccine
Expert reaction to reports that Ireland has suspended the use of the Oxford-AstraZeneca vaccine
“Since we know with great certainty that the vaccine prevents Covid-19 with its attendant disease, and we are almost totally uncertain that the vaccine can have caused this problem, the risk and benefit balance is still very much in favour of the vaccine ...”
Tom Batchelor@_tombatchelor
3/14/2021
The rollout of the AstraZeneca Covid vaccine has been suspended in the Republic of Ireland following a recommendation by Irish health officials after reports of blood-clotting incidents elsewhere in Europe.
Serious blood-clotting events have been recorded after inoculations in Norway, where the AstraZeneca vaccine programme has been paused. Denmark and Iceland have also suspended the rollout pending an investigation.
In an email to The Independent, a spokesperson for the Irish Department of Health said administration of the AstraZeneca jab had been “temporarily deferred” as of Sunday morning.
“The European Medicines Agency (EMA) is already investigating a number of reports of thromboembolic (blood-clotting) events following vaccination with Covid-19 vaccine AstraZeneca,” the spokesperson added. “Further information is expected from the EMA in the next few days, which will include a review of these additional events.
Ireland’s Health Service Executive (HSE) also confirmed it would not be further administering the AstraZeneca vaccine until advised to do so. Other vaccinations are unaffected.
The decision came after Ireland’s National Immunisation Advisory Committee (NIAC) said the rollout should be paused.
Ireland’s deputy chief medical officer, Dr Ronan Glynn, said: “This recommendation has been made following a report from the Norwegian Medicines Agency of four new reports of serious blood-clotting events in adults after vaccination with Covid-19 vaccine AstraZeneca.
“It has not been concluded that there is any link between Covid-19 vaccine AstraZeneca and these cases.
“However, acting on the precautionary principle, and pending receipt of further information, the National Immunisation Advisory Committee has recommended the temporary deferral of the Covid-19 vaccine AstraZeneca vaccination programme in Ireland.”
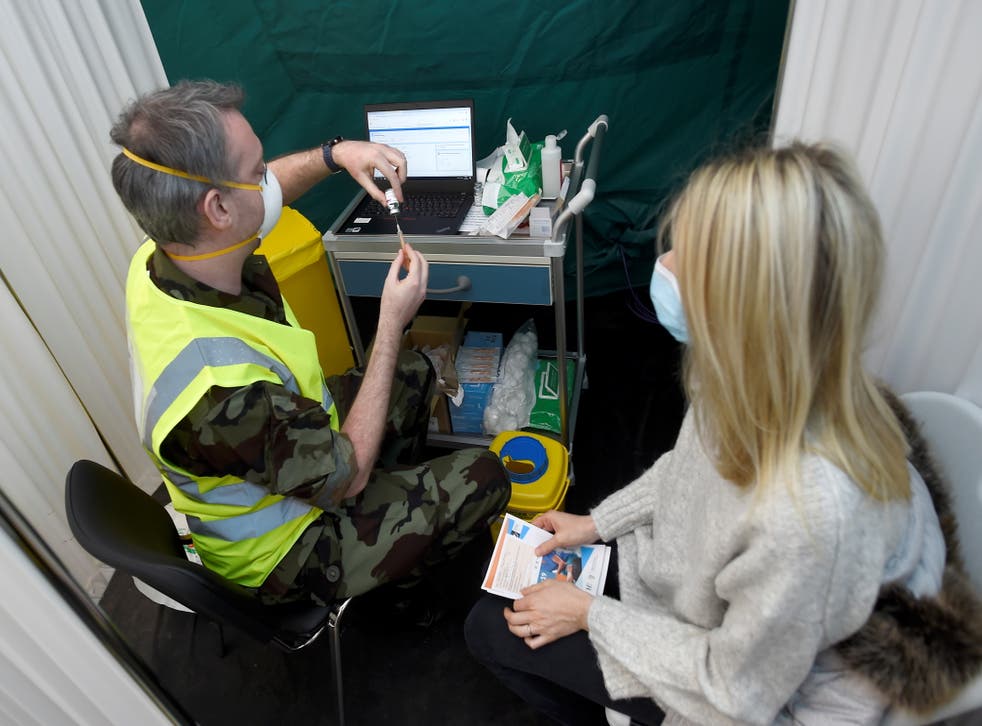
A vaccinator in Dublin prepares a dose of the AstraZeneca jab
(Reuters)
Professor Karina Butler, chair of the NIAC, called it a “precautionary move” and said Irish health authorities would “continue to monitor the situation and if we can be satisfied that these events are coincidental and not caused by this vaccine we will reassess the situation”.
Top Articles
She added: “This vaccine is proven to be very effective against severe Covid-19 disease, which is associated with a risk of clotting events. We have taken this step out of an abundance of caution.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) said it was “aware” of the action in Ireland.
“We are closely reviewing reports but, given the large number of doses administered and the frequency at which blood clots can occur naturally, the evidence available does not suggest the vaccine is the cause,” the body added.
Dr Phil Bryan, its vaccines safety lead, said people "should still go and get their Covid-19 vaccine when asked to do so".
Meanwhile, Ireland’s Health Products Regulatory Authority (HPRA) said it had received a small number of reports associated with blood clots following vaccination with the AstraZeneca vaccine.
They were not as serious as those described in Norway and the number involved was extremely low.
A spokesperson for AstraZeneca said: “An analysis of our safety data that covers reported cases from more than 17 million doses of vaccine administered has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia with Covid-19 vaccine AstraZeneca.
“In fact, the reported numbers of these types of events for Covid-19 vaccine AstraZeneca are not greater than the number that would have occurred naturally in the unvaccinated population.
“In clinical trials, no trends or patterns were observed with regard to pulmonary embolism, deep vein thrombosis, or events possibly related to thrombocytopenia.
“A careful review of all available safety data including these events is ongoing and AstraZeneca is committed to sharing information without delay.”
The recommendation comes after Irish authorities had been pressing the pharmaceutical firm to speed up its supplies to the Republic. More than 117,000 AstraZeneca doses have been administered so far in Ireland.
The EMA reported that one person in Austria was diagnosed with blood clots and died 10 days after vaccination, but it stressed there is “currently no indication that vaccination has caused these conditions”.
Italy has followed Austria, Estonia, Latvia, Luxembourg and Lithuania in banning jabs from one particular batch of 1 million AstraZeneca vaccines, which was sent to 17 countries, after reports of a death.
Later on Sunday, the Netherlands also suspended its AstraZeneca rollout. The Dutch government said in a statement the vaccine will not be used until at least 29 March and it was reported that health authorities would be forced to cancel more than 40,000 appointments.
Another person was admitted to hospital in Austria with pulmonary embolism (blockage in arteries in the lungs) after being vaccinated, while one death involving a blood clot was reported in Denmark.
SPECULATION NOT FACT
A 50-year-old man is also thought to have died in Italy from deep vein thrombosis (DVT), while there has been an unconfirmed report of another death in Italy.
Commenting on the decision to pause the AstraZeneca rollout in Denmark and Norway, Stephen Evans, a professor at the London School of Hygiene and Tropical Medicine, said earlier this week that it was a “super-cautious approach based on some isolated reports in Europe”.
He added: “Since we know with great certainty that the vaccine prevents Covid-19 with its attendant disease, and we are almost totally uncertain that the vaccine can have caused this problem, the risk and benefit balance is still very much in favour of the vaccine in my view.
Our company
Careers
Investors
Media
Sustainability
Partnering
Update on the safety of COVID-19 Vaccine AstraZeneca
Following a recent concern raised around thrombotic events, AstraZeneca would like to offer its reassurance on the safety of its COVID-19 vaccine based on clear scientific evidence. Safety is of paramount importance and the Company is continually monitoring the safety of its vaccine.
A careful review of all available safety data of more than 17 million people vaccinated in the European Union (EU) and UK with COVID-19 Vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis (DVT) or thrombocytopenia, in any defined age group, gender, batch or in any particular country.
So far across the EU and UK, there have been 15 events of DVT and 22 events of pulmonary embolism reported among those given the vaccine, based on the number of cases the Company has received as of 8 March. This is much lower than would be expected to occur naturally in a general population of this size and is similar across other licensed COVID-19 vaccines. The monthly safety report will be made public on the European Medicines Agency website in the following week, in line with exceptional transparency measures for COVID-19.
Furthermore, in clinical trials, even though the number of thrombotic events was small, these were lower in the vaccinated group. There has also been no evidence of increased bleeding in over 60,000 participants enrolled.
Ann Taylor, Chief Medical Officer, said: “Around 17 million people in the EU and UK have now received our vaccine, and the number of cases of blood clots reported in this group is lower than the hundreds of cases that would be expected among the general population. The nature of the pandemic has led to increased attention in individual cases and we are going beyond the standard practices for safety monitoring of licensed medicines in reporting vaccine events, to ensure public safety.”
In terms of quality, there are also no confirmed issues related to any batch of our vaccine used across Europe, or the rest of the world. Additional testing has, and is, being conducted by ourselves and independently by European health authorities and none of these re-tests have shown cause for concern. During the production of the vaccine more than 60 quality tests are conducted by AstraZeneca, its partners and by more than 20 independent testing laboratories. All tests need to meet stringent criteria for quality control and this data is submitted to regulators within each country or region for independent review before any batch can be released to countries.
The safety of the public will always come first. The Company is keeping this issue under close review but available evidence does not confirm that the vaccine is the cause. To overcome the pandemic, it is important that people get vaccinated when invited to do so.
COVID-19 Vaccine AstraZeneca, formerly AZD1222
COVID-19 Vaccine AstraZeneca was co-invented by the University of Oxford and its spin-out company, Vaccitech. It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body.
The vaccine has been granted a conditional marketing authorisation or emergency use in more than 70 countries across six continents, and with the recent Emergency Use Listing granted by the World Health Organization accelerates the pathway to access in up to 142 countries through the COVAX Facility.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas - Oncology, Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
Europe 02:49, 16-Mar-2021
Experts have cautioned that while we shouldn't be "too worried" about taking the Oxford University-AstraZeneca vaccine, governments should be concerned that a ban on the jab may cause more long-term vaccine hesitancy.
The warning comes as a slew of European countries, including Germany, the Netherlands and France, suspended the UK-made jab after reports the drug was linked to "serious blood clotting in adults."
However, Lawrence Young, a professor of molecular oncology at Warwick University, told CGTN Europe that despite the reports, there was little to worry about: "I don't think any of us actually have any concerns that this is a dangerous vaccine."
"We've seen all the data that's come out from the clinical trials that demonstrate this vaccine, along with others, is safe," he said, pointing to the fact the UK and the EU's regulators had already signed off on the jab.
"It's inevitable that there are going to be some bumps in the road and, of course, one has to be always concerned about safety and monitoring safety," he said. But stressed: "I don't think we should be too worried."
European nations initially started suspending single batches of the AstraZeneca vaccine in early March. However, after several reports of blood clotting and deaths in people who had taken the jab, some countries started enforcing a temporary ban.
But Young, an internationally renowned virus specialist who has recently been developing tests to detect COVID-19 antigens and antibodies, said it was vital to compare any adverse effects of vaccines with those that normally occur in the general population.
That was particularly true, he said, when blood clots were already a regular complication of COVID-19 and even prior to the pandemic, were "sadly, very, very common."
"In the UK alone, there are at least 3,000 reported cases of blood clot-associated diseases a month," said the professor. "When you're vaccinating millions of people, you're going to see a similar trend in those individuals."
However, he expressed concern that the decision to suspend AstraZeneca could cause serious problems, given the fact that in Europe "there are many folk who are vaccine hesitant."
He added: "I think it's really important that we report these differences, these adverse effects responsibly, but also stress the fact that overall, the safety data for this vaccine and the other vaccines are very, very good indeed."
Our current COVID-19 vaccines are "much safer than lots of over-the-counter drugs that people take every day," he added, saying that if AstraZeneca's bad PR didn't stop, it would negatively impact Europe's vaccine programs.
"This is one of the front-runner vaccines, not least because it's cheap, and also we know it's very effective," said Lawrence.
"We've heard a lot through the pandemic about 'none of us are safe until all of us are safe,'" he added in reference to the need for mass-vaccination.

No comments:
Post a Comment