
Oxford’s Covid-19 vaccine is safe and effective, further data shows
NINA MASSEY, PA SCIENCE CORRESPONDENT
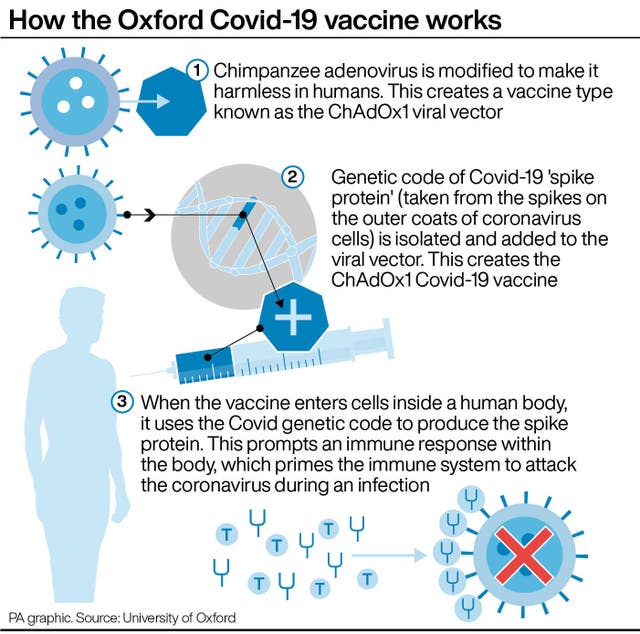
The University of Oxford’s coronavirus vaccine induces an immune response and is safe, according to more data published by researchers.
The UK has secured 100 million doses of the jab which the university is developing with pharmaceutical giant AstraZeneca.
And the Medicines and Healthcare products Regulatory Agency is currently considering whether or not to grant approval for the vaccine to be rolled out.
The research published on Thursday includes data from phase one/two clinical trials of the ChAdOx1 nCoV-19 Covid-19 vaccine, and shows why the team decided to move to a two-dose regimen in ongoing phase three trials.
The data also shows how the vaccine, developed with AstraZeneca, induces broad antibody and T cell functions.
Professor Katie Ewer at the University of Oxford said: “This highly detailed analysis of the immune responses to ChAdOx1 nCoV-19 further underpins the potential of this vaccine to induce protection against Covid-19 disease and provides additional reassurance of the safety of this approach.
“Using these advanced immunological techniques, we can better understand the different cellular and antibody-mediated mechanisms that contribute to the protection afforded by this vaccine, as demonstrated in the recent data from the subsequent phase three trials.”
Previous studies have shown that in order to develop any vaccine against Sars-CoV-2 coronavirus, two key elements of the immune system need to be activated.
These involve stimulating antibodies against the coronavirus spike protein, as well as robust T cell responses.
The findings are reported in two papers, both released in the journal Nature Medicine.
One paper outlines the early-stage planning involved in the design of phase trials to investigate two booster dose schedules – a standard dose followed by a second standard dose and a standard dose followed by a lower dose.
Researchers used data from this to support the change to a two-dose regimen in the ongoing phase three trials.
The booster doses of the vaccine induced stronger antibody responses than a single dose, with the standard dose/standard dose activating the best response – supporting the decision to move to a two-dose vaccine regimen in phase three trials.
The paper also shows that many different antibody functions are triggered by the vaccine that may be important in protecting against the disease.
In the second paper, the authors detail an investigation of the T cell and antibody responses generated by the vaccine.
The authors report induction of a T cell subset, known to be particularly effective at clearing virus-infected cells from the body during infection.
This type of T cell response in combination with the detailed antibody profile is highly favourable for an efficacious vaccine, and further supports the profile of this vaccine as safe, researchers say.




 Credit: CC0 Public Domain
Credit: CC0 Public Domain
 Credit: National Research Council of Science & Technology
Credit: National Research Council of Science & Technology






