We thought COVID-19 was just a respiratory virus—we were wrong
by Ariel Bleicher, Katherine Conrad,
University of California, San Francisco JULY 31,2020
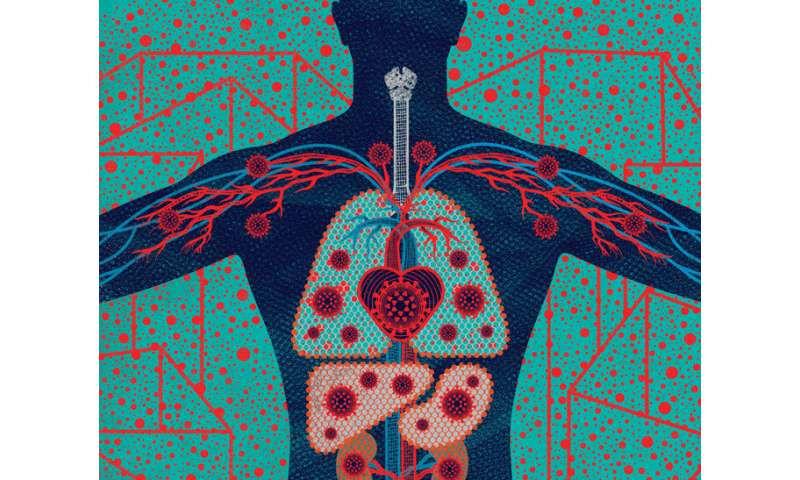

Credit: Anna and Elena Balbusso
In late January, when hospitals in the United States confirmed the presence of the novel coronavirus, health workers knew to watch for precisely three symptoms: fever, cough, and shortness of breath. But as the number of infections climbed, the symptom list began to grow.
Some patients lost their sense of smell and taste. Some had nausea or diarrhea. Some had arrhythmias or even heart attacks. Some had damaged kidneys or livers. Some had headaches, blood clots, rashes, swelling, or strokes. Many had no symptoms at all.
By June, clinicians were swapping journal papers, news stories, and tweets describing more than three dozen ways that COVID-19, the disease the coronavirus causes, appears to manifest itself. Now researchers at UC San Francisco and around the world have begun taking a closer look at this dizzying array of symptoms to get at the disease's root causes. They are learning from people inside the hospital and out; people on the brink of death and only mildly sick; people newly exposed and recovered; people young and old, Black, brown, and white. And they are beginning to piece together the story of a virus unlike any known before.
How infection sets in
Viruses lead a curious purgatorial existence of being neither fully alive nor dead. Enveloped in a protein cloak, a virus consists almost entirely of genetic material—DNA or RNA, the blueprints for all of life. But it can't reproduce on its own. To survive, it must break into a cell and co-opt the cell's gene-copying machinery.
The novel coronavirus, an RNA virus named SARS-CoV-2, has become notorious for its skill at breaking and entering human cells. Its tools of choice are the protein spikes protruding from its surface—a feature that distinguishes all coronaviruses. The spikes of SARS-CoV-2 are the crème de la crème: By the luck of the evolutionary draw, they are able to easily grab hold of protein gates on human cells known as ACE2 receptors and, like jackknives, pry these gates open.
"You can think of an ACE2 receptor like a docking site," says Faranak Fattahi, Ph.D., a UCSF Sandler Fellow. When the coronavirus pandemic hit San Francisco, Fattahi repurposed her laboratory to study this key receptor, which normally plays a role in regulating blood pressure. "When the virus lands on it," she says, "it initiates a molecular process that brings the virus inside the cell."
If you're exposed to SARS-CoV-2—say, from a cough or sneeze—the virus will likely first encounter ACE2 receptors on cells in your nose or throat. But these receptors also populate your heart, gut, and other organs. Fattahi's team has found evidence suggesting that male sex hormones such as testosterone may increase the number of ACE2 receptors that cells produce, which could help explain why SARS-CoV-2 seems to wreak greater havoc on men than on women and why kids rarely get sick. "The fewer ACE2 receptors, the less risk of infection—that's the idea," she says, adding that this hypothesis for the disease's gender gap is only one of several.
Once inside a few initial host cells, the virus sets them to work churning out copies of itself. Within hours, thousands of new virus particles begin bursting forth, ready to infect more cells. Although SARS-CoV-2 is less deadly than the original SARS virus, which emerged in 2002, it replicates more rapidly. Also unlike SARS, which primarily infects the lungs, SARS-CoV-2 replicates throughout the airway, including in the nose and throat, making it highly contagious—like the common cold.
However, infection with SARS-CoV-2 usually doesn't feel like a cold. Fewer than 20 percent of infected people who eventually show up at a hospital report having had a sore throat or runny nose. During the first few days of being infected, you're more likely to have a fever, dry cough or, peculiarly, lose your sense of smell or taste.
Most likely, though, you won't feel sick at all. When UCSF researchers tested people for SARS-CoV-2 in San Francisco's Mission District, 53 percent of those infected never had any symptoms. "That's much higher than expected," says Monica Gandhi, M.D., MPH, a UCSF professor of medicine with expertise in HIV. Surveys of outbreaks in nursing homes and prisons show similar or even higher numbers. "If we did a mass testing campaign on 300 million Americans right now, I think the rate of asymptomatic infection would be somewhere between 50 percent and 80 percent of cases," Gandhi says. Millions of people may be spreading the virus without knowing it, she points out, making asymptomatic transmission the Achilles' heel of efforts to control the pandemic—and highlighting the importance of universal masking.
In late January, when hospitals in the United States confirmed the presence of the novel coronavirus, health workers knew to watch for precisely three symptoms: fever, cough, and shortness of breath. But as the number of infections climbed, the symptom list began to grow.
Some patients lost their sense of smell and taste. Some had nausea or diarrhea. Some had arrhythmias or even heart attacks. Some had damaged kidneys or livers. Some had headaches, blood clots, rashes, swelling, or strokes. Many had no symptoms at all.
By June, clinicians were swapping journal papers, news stories, and tweets describing more than three dozen ways that COVID-19, the disease the coronavirus causes, appears to manifest itself. Now researchers at UC San Francisco and around the world have begun taking a closer look at this dizzying array of symptoms to get at the disease's root causes. They are learning from people inside the hospital and out; people on the brink of death and only mildly sick; people newly exposed and recovered; people young and old, Black, brown, and white. And they are beginning to piece together the story of a virus unlike any known before.
How infection sets in
Viruses lead a curious purgatorial existence of being neither fully alive nor dead. Enveloped in a protein cloak, a virus consists almost entirely of genetic material—DNA or RNA, the blueprints for all of life. But it can't reproduce on its own. To survive, it must break into a cell and co-opt the cell's gene-copying machinery.
The novel coronavirus, an RNA virus named SARS-CoV-2, has become notorious for its skill at breaking and entering human cells. Its tools of choice are the protein spikes protruding from its surface—a feature that distinguishes all coronaviruses. The spikes of SARS-CoV-2 are the crème de la crème: By the luck of the evolutionary draw, they are able to easily grab hold of protein gates on human cells known as ACE2 receptors and, like jackknives, pry these gates open.
"You can think of an ACE2 receptor like a docking site," says Faranak Fattahi, Ph.D., a UCSF Sandler Fellow. When the coronavirus pandemic hit San Francisco, Fattahi repurposed her laboratory to study this key receptor, which normally plays a role in regulating blood pressure. "When the virus lands on it," she says, "it initiates a molecular process that brings the virus inside the cell."
If you're exposed to SARS-CoV-2—say, from a cough or sneeze—the virus will likely first encounter ACE2 receptors on cells in your nose or throat. But these receptors also populate your heart, gut, and other organs. Fattahi's team has found evidence suggesting that male sex hormones such as testosterone may increase the number of ACE2 receptors that cells produce, which could help explain why SARS-CoV-2 seems to wreak greater havoc on men than on women and why kids rarely get sick. "The fewer ACE2 receptors, the less risk of infection—that's the idea," she says, adding that this hypothesis for the disease's gender gap is only one of several.
Once inside a few initial host cells, the virus sets them to work churning out copies of itself. Within hours, thousands of new virus particles begin bursting forth, ready to infect more cells. Although SARS-CoV-2 is less deadly than the original SARS virus, which emerged in 2002, it replicates more rapidly. Also unlike SARS, which primarily infects the lungs, SARS-CoV-2 replicates throughout the airway, including in the nose and throat, making it highly contagious—like the common cold.
However, infection with SARS-CoV-2 usually doesn't feel like a cold. Fewer than 20 percent of infected people who eventually show up at a hospital report having had a sore throat or runny nose. During the first few days of being infected, you're more likely to have a fever, dry cough or, peculiarly, lose your sense of smell or taste.
Most likely, though, you won't feel sick at all. When UCSF researchers tested people for SARS-CoV-2 in San Francisco's Mission District, 53 percent of those infected never had any symptoms. "That's much higher than expected," says Monica Gandhi, M.D., MPH, a UCSF professor of medicine with expertise in HIV. Surveys of outbreaks in nursing homes and prisons show similar or even higher numbers. "If we did a mass testing campaign on 300 million Americans right now, I think the rate of asymptomatic infection would be somewhere between 50 percent and 80 percent of cases," Gandhi says. Millions of people may be spreading the virus without knowing it, she points out, making asymptomatic transmission the Achilles' heel of efforts to control the pandemic—and highlighting the importance of universal masking.

Spikes on the virus’s surface act like jackknives to break and enter human cells. Credit: UCSF
"The majority of people who have COVID-19 are out in the community, and they are either asymptomatic or only mildly ill," says Sulggi Lee, M.D., Ph.D., a UCSF assistant professor of medicine. When the coronavirus pandemic hit San Francisco in early March, Lee conceived a study to investigate why. She scrambled to assemble a team and procure funding and equipment. She borrowed a colleague's mobile clinic—a van outfitted with an exam table and a phlebotomy chair—so that her team could drive around the city, collecting samples from infected people. Lee hopes data from the study, called CHIRP (COVID-19 Host Immune Response Pathogenesis), will show how people's immune systems respond as SARS-CoV-2 starts to gain a foothold in their bodies.
"A lot is riding on that initial response," she says. If Lee and her collaborators can figure out the biological processes that allow some infected people to stay relatively well, they can perhaps use that knowledge to prevent others from falling severely ill.
Battling in the lungs
True to its name, SARS-CoV-2 (which stands for severe acute respiratory syndrome coronavirus 2) is first and foremost a bad respiratory virus. If your immune system doesn't defeat it at its landing site in your nose or throat, it will advance down your windpipe, infiltrating the cells lining your lungs' branching air tubes. At the tubes' ends, tiny air sacs called alveoli pass oxygen to your blood. As the virus multiplies, the alveoli may fill with fluid, shutting down this critical gas exchange. Your blood-oxygen level may drop and, typically about six days into an infection, you may start feeling short of breath.
What causes this mayhem? "Some of it is definitely caused by the virus itself," says Michael Matthay, M.D., a UCSF professor of medicine who has studied acute respiratory diseases for more than 30 years. Inevitably, a fast-replicating virus will kill or injure many of the lung cells it infects; the more cells it infects, the more ruin it will leave in its wake.
But SARS-CoV-2 doesn't appear to be a savage destroyer of cells. Although it's too early to know for sure, the virus's fatality rate seems to be roughly 10 times that of the flu. "You would think that's because it's just a killing machine," says Max Krummel, Ph.D., UCSF's Smith Professor of Experimental Pathology and chair of the Bakar ImmunoX initiative. So far, however, the science suggests otherwise.
"One of the weirder things about this new coronavirus is it doesn't seem to be incredibly cytopathic, by which we mean cell-killing," Krummel says. "Flu is really cytopathic; if you add it to human cells in a petri dish, the cells burst within 18 hours." But when UCSF researchers subjected human cells to SARS-CoV-2, many of the infected cells never perished. "It's pretty compelling data that maybe we're not dealing with a hugely aggressive virus," Krummel says.
The bigger provocation, he suspects, may be your own immune system. Like any pathogen, SARS-CoV-2 will trigger an immune attack within minutes of entering your body. This counterstrike is extraordinarily complex, involving many tactics, cells, and molecules. In a UCSF study called COMET (COVID-19 Multi-Phenotyping for Effective Therapies), Krummel and other scientists have been observing this immune warfare in more than 30 people admitted to UCSF hospitals with COVID-19 and other respiratory infections. "What we're doing is looking at patients' blood, their genes, and the secretions from their noses and lungs, and we're asking, 'What's your army? What's your response strategy?'"
An early analysis of COMET data, Krummel says, suggests that the immune systems of many hospitalized patients mobilize differently—and more aggressively—against SARS-CoV-2 than against influenza viruses, which cause the flu. Their lungs are ravaged, these data suggest, not by the virus alone but by the detritus of an immunological battle gone awry. This rogue immune response could explain why, around day 11 of a COVID-19 infection, patients often develop a severe pneumonia known as acute respiratory distress syndrome, or ARDS.
Ultimately, COMET seeks to find COVID-19 therapies that can rein in an overeager immune system in order to prevent or treat ARDS. But that feat won't be easy, says Carolyn Calfee, M.D., MAS '09, an ARDS expert, UCSF professor of medicine, and co-leader of the study. Too much or the wrong kind of intervention, she explains, could cripple a person's immune system to the point where it can't clear an infection. "It's a fine line between therapeutic and deleterious," Calfee says. "We're trying to find that balance."
Typically, people who die from COVID-19 ARDS die around day 19. Reported rates of mortality have varied widely, with the highest rates being where the pandemic has hit hardest, overwhelming hospital resources and staff. At UCSF hospitals—likely due to the city's early shelter-in-place orders, which prevented an initial surge of COVID-19 cases—so far only 10 of 85 critically ill patients have died.

"The majority of people who have COVID-19 are out in the community, and they are either asymptomatic or only mildly ill," says Sulggi Lee, M.D., Ph.D., a UCSF assistant professor of medicine. When the coronavirus pandemic hit San Francisco in early March, Lee conceived a study to investigate why. She scrambled to assemble a team and procure funding and equipment. She borrowed a colleague's mobile clinic—a van outfitted with an exam table and a phlebotomy chair—so that her team could drive around the city, collecting samples from infected people. Lee hopes data from the study, called CHIRP (COVID-19 Host Immune Response Pathogenesis), will show how people's immune systems respond as SARS-CoV-2 starts to gain a foothold in their bodies.
"A lot is riding on that initial response," she says. If Lee and her collaborators can figure out the biological processes that allow some infected people to stay relatively well, they can perhaps use that knowledge to prevent others from falling severely ill.
Battling in the lungs
True to its name, SARS-CoV-2 (which stands for severe acute respiratory syndrome coronavirus 2) is first and foremost a bad respiratory virus. If your immune system doesn't defeat it at its landing site in your nose or throat, it will advance down your windpipe, infiltrating the cells lining your lungs' branching air tubes. At the tubes' ends, tiny air sacs called alveoli pass oxygen to your blood. As the virus multiplies, the alveoli may fill with fluid, shutting down this critical gas exchange. Your blood-oxygen level may drop and, typically about six days into an infection, you may start feeling short of breath.
What causes this mayhem? "Some of it is definitely caused by the virus itself," says Michael Matthay, M.D., a UCSF professor of medicine who has studied acute respiratory diseases for more than 30 years. Inevitably, a fast-replicating virus will kill or injure many of the lung cells it infects; the more cells it infects, the more ruin it will leave in its wake.
But SARS-CoV-2 doesn't appear to be a savage destroyer of cells. Although it's too early to know for sure, the virus's fatality rate seems to be roughly 10 times that of the flu. "You would think that's because it's just a killing machine," says Max Krummel, Ph.D., UCSF's Smith Professor of Experimental Pathology and chair of the Bakar ImmunoX initiative. So far, however, the science suggests otherwise.
"One of the weirder things about this new coronavirus is it doesn't seem to be incredibly cytopathic, by which we mean cell-killing," Krummel says. "Flu is really cytopathic; if you add it to human cells in a petri dish, the cells burst within 18 hours." But when UCSF researchers subjected human cells to SARS-CoV-2, many of the infected cells never perished. "It's pretty compelling data that maybe we're not dealing with a hugely aggressive virus," Krummel says.
The bigger provocation, he suspects, may be your own immune system. Like any pathogen, SARS-CoV-2 will trigger an immune attack within minutes of entering your body. This counterstrike is extraordinarily complex, involving many tactics, cells, and molecules. In a UCSF study called COMET (COVID-19 Multi-Phenotyping for Effective Therapies), Krummel and other scientists have been observing this immune warfare in more than 30 people admitted to UCSF hospitals with COVID-19 and other respiratory infections. "What we're doing is looking at patients' blood, their genes, and the secretions from their noses and lungs, and we're asking, 'What's your army? What's your response strategy?'"
An early analysis of COMET data, Krummel says, suggests that the immune systems of many hospitalized patients mobilize differently—and more aggressively—against SARS-CoV-2 than against influenza viruses, which cause the flu. Their lungs are ravaged, these data suggest, not by the virus alone but by the detritus of an immunological battle gone awry. This rogue immune response could explain why, around day 11 of a COVID-19 infection, patients often develop a severe pneumonia known as acute respiratory distress syndrome, or ARDS.
Ultimately, COMET seeks to find COVID-19 therapies that can rein in an overeager immune system in order to prevent or treat ARDS. But that feat won't be easy, says Carolyn Calfee, M.D., MAS '09, an ARDS expert, UCSF professor of medicine, and co-leader of the study. Too much or the wrong kind of intervention, she explains, could cripple a person's immune system to the point where it can't clear an infection. "It's a fine line between therapeutic and deleterious," Calfee says. "We're trying to find that balance."
Typically, people who die from COVID-19 ARDS die around day 19. Reported rates of mortality have varied widely, with the highest rates being where the pandemic has hit hardest, overwhelming hospital resources and staff. At UCSF hospitals—likely due to the city's early shelter-in-place orders, which prevented an initial surge of COVID-19 cases—so far only 10 of 85 critically ill patients have died.

SARS-CoV-2 replicates throughout the airway, making it highly contagious, like the common cold. Credit: Anna and Elena Balbusso/UCSF
"The good news is that we've been doing clinical trials of best-care practices for ARDS since 1998," Matthay says. Thanks to research by him and others, for example, clinicians have long known which ventilator settings result in the fewest deaths and how to flip patients onto their stomachs—a technique known as proning—to best help them breathe. If public health measures can keep hospital admissions low so that frontline providers can make good use of the skills and knowledge they already have, we may find that we have less to fear from SARS-CoV-2 than we thought.
On the other hand, the virus behaves in ways that are still mysterious.
Heart failure
In April, Susan Parson, M.D., a Bay Area medical examiner, made a startling discovery. For nearly two months, officials had believed that the first people in the U.S. to die from COVID-19 had died of respiratory failure in Washington state in late February. At the time, the U.S. Centers for Disease Control and Prevention limited testing to people who had respiratory symptoms and had recently traveled to China or otherwise been exposed to the virus. Those restrictions, however, turned out to be misguided.
As a medical examiner for California's Santa Clara County, Parson had done a routine autopsy on a 57-year-old woman named Patricia Dowd, who had died suddenly at home on February 6. In Dowd's tissues, Parson found the cause of her death: SARS-CoV-2. But the virus hadn't wrecked Dowd's lungs. In fact, she had only mild pneumonia. Instead, SARS-CoV-2 had ruptured her heart.
Meanwhile, epidemiologists began learning that preexisting heart disease and related conditions put people at greater risk of suffering and dying from COVID-19. "We're finding that many patients who have more severe forms of the illness are obese, they are diabetic, they are hypertensive," says cardiologist Nisha Parikh, M.D., a UCSF associate professor who specializes in population health research. Such risk factors, she says, are unusual. "They're not ones that really stood out in prior epidemics."
Clinicians, too, were seeing surprising numbers of COVID-19 patients develop heart problems—muscle weakness, inflammation, arrhythmias, even heart attacks. "We're not used to respiratory viruses having such dire consequences on the heart in such apparently high numbers," says cardiologist Gregory Marcus, M.D., MAS '08, UCSF's Endowed Professor of Atrial Fibrillation Research. Many patients whose hearts acted up also had failing lungs. But others had no other symptoms or, like Dowd, only mild ones.
Since March, Marcus has co-led one of the largest community surveys to better understand the spread of SARS-CoV-2 and its myriad effects. The study, dubbed COVID-19 Citizen Science, has so far enrolled more than 27,000 people; anyone with a smartphone can participate. Marcus plans to also start collecting data from wearable devices, including Fitbits and Zio patches, which wirelessly monitor heart rhythms. "There may be large numbers of people who are suffering from cardiovascular effects of COVID-19 in the absence of other symptoms," Marcus says. "I'm worried we're missing those cases."
It stands to reason that SARS-CoV-2 affects the heart. After all, heart cells are flush with ACE2 receptors, the virus's vital port of entry. And, indeed, laboratory experiments suggest that the virus can enter and replicate in cultured human heart cells, says Bruce Conklin, M.D., a professor of medicine and an expert in heart-disease genetics at UCSF and the Gladstone Institutes.
But Conklin doesn't think SARS-CoV-2 necessarily kills heart cells outright. Rather, in the process of copying itself, the virus steals pieces of the genetic instructions that tell the heart cells how to do their job. "It's hauling away and hijacking stuff that's necessary for the heart to beat," he says. He is currently testing this hypothesis using human heart cells grown in cup-sized vessels in the lab of Todd McDevitt, Ph.D., a bioengineer at UCSF and the Gladstone Institutes.
It's also possible, however, that an infected person's own immune system may do the majority of the damage in the heart, as it appears to do in the lungs. "The heart probably gets infected by a lot of other viruses, and they don't have a lethal effect," Conklin says. "What makes this one different?"
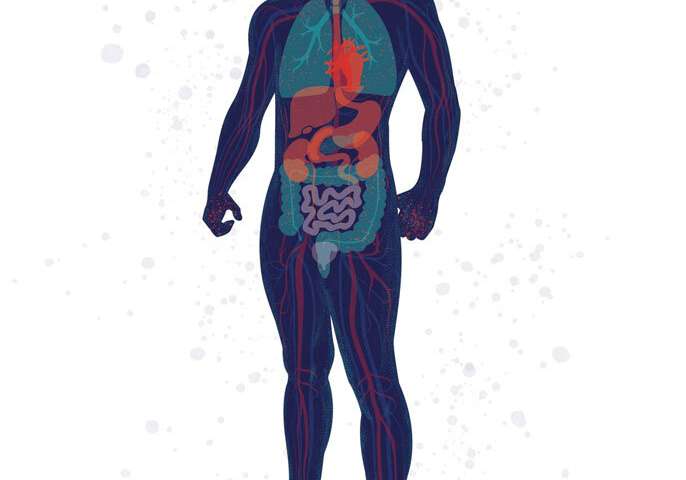 From Head to “COVID Toes”: People with COVID-19 exhibit from none to many of these symptoms. Some symptoms (such as fever, cough, and loss of smell) are common, while others (such as sore throat, pink eye, and stroke) are rare. Credit: Illustration: Stephanie Koch. Concept: Jennifer Babik, M.D., Ph.D.
From Head to “COVID Toes”: People with COVID-19 exhibit from none to many of these symptoms. Some symptoms (such as fever, cough, and loss of smell) are common, while others (such as sore throat, pink eye, and stroke) are rare. Credit: Illustration: Stephanie Koch. Concept: Jennifer Babik, M.D., Ph.D.
Stranger things
Toward the end of March, as San Francisco began to warm up, Sonia got cold feet. She put on wool socks and turned up her heater. Still, her feet felt frozen. Three days later, her soles turned splotchy purple. Red dots appeared on her toes. At night, her cold feet itched and burned. Walking hurt. And she was exhausted, napping through afternoon Zoom meetings. "It was so bizarre," says Sonia, a San Francisco resident. A week later, her symptoms were gone.
"Yes, COVID," wrote Lindy Fox, M.D., a UCSF professor of dermatology, replying to an email describing Sonia's case. Sonia wasn't surprised. Anyone, like her, who's been following news of the pandemic has probably heard about "COVID toes," a painful or itchy skin rash that sometimes pops up in young adults with otherwise mild or asymptomatic cases of COVID-19. "It looks like what we call pernio, or chilblains," Fox says, "which is a pretty common phenomenon when somebody goes out in cold weather—they start to get purple or pink bumps on their fingers or toes."
Many people with rashes like Sonia's don't test positive for COVID-19, Fox says, which has made some clinicians skeptical of the connection; when patients have both, it's just a coincidence, they believe. But Fox doesn't think so. For one thing, "the time of year is wrong," she says. "Pernio usually shows up in the dead of winter." Even more compelling, dermatologists around the world are "getting crazy numbers of calls about it," Fox says. "In the last three weeks, I've had somewhere between 10 and 12 patients.
Normally, I have four a year."
And it's not just dermatologists who are adding their observations to COVID-19's ever-expanding symptom list. Gut specialists are finding that 20 percent to 40 percent of people with the disease experience diarrhea, nausea, or vomiting before other symptoms, says gastroenterologist Michael Kattah, M.D., Ph.D., a UCSF assistant professor. If you swallow virus particles, he says, there's a good chance they will infect cells lining your stomach, small intestine, or colon. As in the lungs and heart, these cells are studded with vulnerable ACE2 portals.
Especially disconcerting, Kattah says, is how long the virus seems to persist in the gut. About 50 percent of patients with COVID-19 have virus particles in their stools, often for weeks after their nose swabs test negative, he points out. Laboratory studies show that these particles are often still alive and can infect cells in a petri dish. Whether fecal transmission occurs between people, however, is an open question. If the answer is yes, people recovering from COVID-19 may need to stay quarantined even after they feel well, and the rest of us will need to be as meticulous about bathroom hygiene as we've become about handwashing and mask-wearing.
Other specialists are also raising flags. Neurologists worry about reports of COVID-19 patients with headaches, "brain fog," loss of the sense of smell, dizziness, delirium, and, in rare cases, stroke. Nephrologists worry about kidney stress and failure. Hepatologists worry about liver injuries. Ophthalmologists worry about pink eye. Pediatricians, meanwhile, worry about a peculiar COVID-related inflammatory syndrome that's showing up in kids and young adults.
Researchers are still sorting out the causes for this constellation of effects. If you come down with a particular symptom, is it because the virus is attacking your cells? Because your immune system is overreacting? Or just because you're very sick? In any severe illness, for example, the kidneys must work extra hard to filter waste and control nutrients and fluid; if overtaxed, they may begin to fail. Similarly, cognitive problems can result from increased blood toxins due to stressed kidneys or from low oxygen due to respiratory distress. "There's a lot of smoke," says Michael Wilson, M.D. '07, MAS '16, the Rachleff Distinguished Professor at UCSF's Weill Institute for Neurosciences. "We need to figure out where the fire is coming from."
Recently, there's been speculation that some of COVID-19's seemingly disparate symptoms may stem from trouble in the blood. Blood clots, for example, are showing up in cases of COVID-19 frequently enough for clinicians to take notice. "There's something unique about the coagulation system in these patients," says nephrologist Kathleen Liu, M.D. '99, Ph.D. '97, MAS '07, a UCSF professor of medicine. In caring for COVID-19 patients on dialysis machines, she's been surprised to see blood clots block dialysis tubes more than usual. Clotted tubes are common, she says, "but this is extreme."
That may be because, as growing evidence suggests, SARS-CoV-2 can infect cells in the walls of blood vessels that help regulate blood flow and coagulation, or clotting. If true, this behavior could explain some of the virus's weirder (and rarer) manifestations, such as heart attacks, strokes, and even "COVID toes."
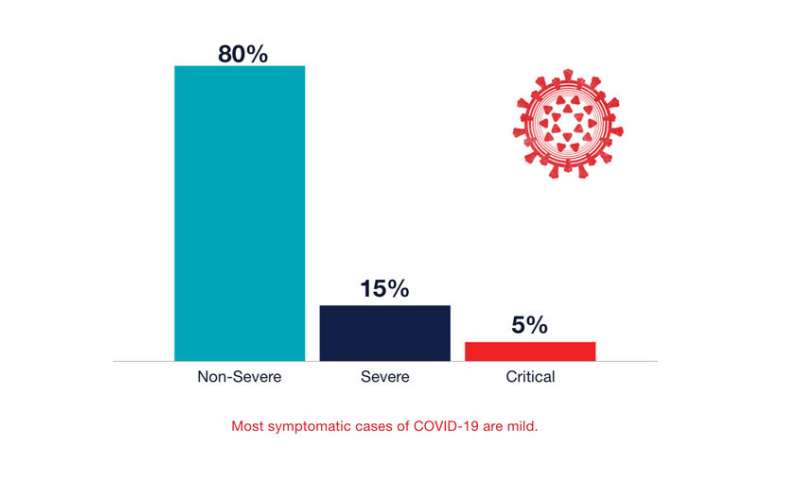 Most symptomatic cases of COVID-19 are mild. Credit: UCSF
Most symptomatic cases of COVID-19 are mild. Credit: UCSF
"Our vasculature is a contiguous system," says cardiologist Parikh. "Thus injury in one area, such as blood vessels in the lungs, can set off clotting cascades that affect multiple organs." Some of that trouble likely results from inflammation triggered by the immune system, she points out, although another culprit may be the body's RAAS, or renin-angiotensin-aldosterone system, a hormone system that controls blood pressure and fluid balance. Because RAAS involves ACE2 receptors, Parikh suspects it may become disrupted when the virus infects cells through these receptors, thus triggering coagulation and other downstream effects. Her lab is now studying this system in COVID-19 patients to better understand how SARS-CoV-2 infection affects it.
Inevitably, some ailments may turn out to be red herrings. During a pandemic, when people are flocking to hospitals with infections, clinicians will also see a rise in other health problems, simply by the rules of statistics, points out S. Andrew Josephson, M.D., the Francheschi-Mitchell Professor, chair of UCSF's neurology department, and a member of the Weill Institute for Neurosciences. "If the prevalence of infection is high, then almost any condition—a broken leg, if you will—you might conclude is associated with COVID-19."
"As clinicians, we want to get information to our medical community and to the public as quickly as possible," Josephson says, "but we have to be cautious about not making too big a deal of a little blip."
The long tail
As with any infection, how long a bout of COVID-19 lasts varies from person to person. If you're ill enough to need critical care, you can expect the disease to take at least a few weeks to run its course. In some cases, symptoms persist for months. For a typical milder case, though, you should feel better within a couple weeks.
At that point, the question foremost on your mind will be: Am I immune? There are now more than a dozen antibody tests on the market, but most are unreliable, according to UCSF research. And even the best tests can't tell you whether you have enough of the right kinds of antibodies to protect you against reinfection. "There is a lot of hope and belief that we'll have an antibody test that actually informs us of immunity, but we're not quite there yet," says Chaz Langelier, M.D., Ph.D., a UCSF assistant professor of medicine who is working to improve diagnostic tools for COVID-19.
What we have in the meantime are a lot of unknowns: If you do become immune to SARS-CoV-2, when and how does that occur? Will you gain immunity from a mild or asymptomatic case, as well as a severe one? How long will that immunity last?
"The answers will have huge implications for social distancing and masking and for getting the economy back up and running," says Michael Peluso, M.D., a clinical fellow who came to UCSF three years ago to help fight HIV. Now he's co-leading a new study called LIINC (Long-term Impact of Infection with Novel Coronavirus), which is enrolling people who have been infected with SARS-CoV-2 and will follow them for two years. Besides illuminating changes in immunity over time, LIINC is investigating chronic effects of infection on the immune system, lungs, heart, brain, blood, and other parts of the body.
"I hope people will recover and immunity will be protective and long-lasting, and that will be that," Peluso says.
It's what we all hope. We hope we will beat an infection swiftly—or, better yet, avoid the virus until there is a vaccine. We hope that if we do fall gravely ill, we will be cared for by the best providers and tended to by people we love. The reality, as we already know, is more complicated. And even if COVID-19 doesn't batter our bodies, the pandemic will surely leave scars—on our psyches, our livelihoods, our institutions, and our health—that we are only beginning to fathom. In truth, we don't know how our cards will fall, as individuals or as a people. Only time—and data—will tell.
"The good news is that we've been doing clinical trials of best-care practices for ARDS since 1998," Matthay says. Thanks to research by him and others, for example, clinicians have long known which ventilator settings result in the fewest deaths and how to flip patients onto their stomachs—a technique known as proning—to best help them breathe. If public health measures can keep hospital admissions low so that frontline providers can make good use of the skills and knowledge they already have, we may find that we have less to fear from SARS-CoV-2 than we thought.
On the other hand, the virus behaves in ways that are still mysterious.
Heart failure
In April, Susan Parson, M.D., a Bay Area medical examiner, made a startling discovery. For nearly two months, officials had believed that the first people in the U.S. to die from COVID-19 had died of respiratory failure in Washington state in late February. At the time, the U.S. Centers for Disease Control and Prevention limited testing to people who had respiratory symptoms and had recently traveled to China or otherwise been exposed to the virus. Those restrictions, however, turned out to be misguided.
As a medical examiner for California's Santa Clara County, Parson had done a routine autopsy on a 57-year-old woman named Patricia Dowd, who had died suddenly at home on February 6. In Dowd's tissues, Parson found the cause of her death: SARS-CoV-2. But the virus hadn't wrecked Dowd's lungs. In fact, she had only mild pneumonia. Instead, SARS-CoV-2 had ruptured her heart.
Meanwhile, epidemiologists began learning that preexisting heart disease and related conditions put people at greater risk of suffering and dying from COVID-19. "We're finding that many patients who have more severe forms of the illness are obese, they are diabetic, they are hypertensive," says cardiologist Nisha Parikh, M.D., a UCSF associate professor who specializes in population health research. Such risk factors, she says, are unusual. "They're not ones that really stood out in prior epidemics."
Clinicians, too, were seeing surprising numbers of COVID-19 patients develop heart problems—muscle weakness, inflammation, arrhythmias, even heart attacks. "We're not used to respiratory viruses having such dire consequences on the heart in such apparently high numbers," says cardiologist Gregory Marcus, M.D., MAS '08, UCSF's Endowed Professor of Atrial Fibrillation Research. Many patients whose hearts acted up also had failing lungs. But others had no other symptoms or, like Dowd, only mild ones.
Since March, Marcus has co-led one of the largest community surveys to better understand the spread of SARS-CoV-2 and its myriad effects. The study, dubbed COVID-19 Citizen Science, has so far enrolled more than 27,000 people; anyone with a smartphone can participate. Marcus plans to also start collecting data from wearable devices, including Fitbits and Zio patches, which wirelessly monitor heart rhythms. "There may be large numbers of people who are suffering from cardiovascular effects of COVID-19 in the absence of other symptoms," Marcus says. "I'm worried we're missing those cases."
It stands to reason that SARS-CoV-2 affects the heart. After all, heart cells are flush with ACE2 receptors, the virus's vital port of entry. And, indeed, laboratory experiments suggest that the virus can enter and replicate in cultured human heart cells, says Bruce Conklin, M.D., a professor of medicine and an expert in heart-disease genetics at UCSF and the Gladstone Institutes.
But Conklin doesn't think SARS-CoV-2 necessarily kills heart cells outright. Rather, in the process of copying itself, the virus steals pieces of the genetic instructions that tell the heart cells how to do their job. "It's hauling away and hijacking stuff that's necessary for the heart to beat," he says. He is currently testing this hypothesis using human heart cells grown in cup-sized vessels in the lab of Todd McDevitt, Ph.D., a bioengineer at UCSF and the Gladstone Institutes.
It's also possible, however, that an infected person's own immune system may do the majority of the damage in the heart, as it appears to do in the lungs. "The heart probably gets infected by a lot of other viruses, and they don't have a lethal effect," Conklin says. "What makes this one different?"
 From Head to “COVID Toes”: People with COVID-19 exhibit from none to many of these symptoms. Some symptoms (such as fever, cough, and loss of smell) are common, while others (such as sore throat, pink eye, and stroke) are rare. Credit: Illustration: Stephanie Koch. Concept: Jennifer Babik, M.D., Ph.D.
From Head to “COVID Toes”: People with COVID-19 exhibit from none to many of these symptoms. Some symptoms (such as fever, cough, and loss of smell) are common, while others (such as sore throat, pink eye, and stroke) are rare. Credit: Illustration: Stephanie Koch. Concept: Jennifer Babik, M.D., Ph.D.Stranger things
Toward the end of March, as San Francisco began to warm up, Sonia got cold feet. She put on wool socks and turned up her heater. Still, her feet felt frozen. Three days later, her soles turned splotchy purple. Red dots appeared on her toes. At night, her cold feet itched and burned. Walking hurt. And she was exhausted, napping through afternoon Zoom meetings. "It was so bizarre," says Sonia, a San Francisco resident. A week later, her symptoms were gone.
"Yes, COVID," wrote Lindy Fox, M.D., a UCSF professor of dermatology, replying to an email describing Sonia's case. Sonia wasn't surprised. Anyone, like her, who's been following news of the pandemic has probably heard about "COVID toes," a painful or itchy skin rash that sometimes pops up in young adults with otherwise mild or asymptomatic cases of COVID-19. "It looks like what we call pernio, or chilblains," Fox says, "which is a pretty common phenomenon when somebody goes out in cold weather—they start to get purple or pink bumps on their fingers or toes."
Many people with rashes like Sonia's don't test positive for COVID-19, Fox says, which has made some clinicians skeptical of the connection; when patients have both, it's just a coincidence, they believe. But Fox doesn't think so. For one thing, "the time of year is wrong," she says. "Pernio usually shows up in the dead of winter." Even more compelling, dermatologists around the world are "getting crazy numbers of calls about it," Fox says. "In the last three weeks, I've had somewhere between 10 and 12 patients.
Normally, I have four a year."
And it's not just dermatologists who are adding their observations to COVID-19's ever-expanding symptom list. Gut specialists are finding that 20 percent to 40 percent of people with the disease experience diarrhea, nausea, or vomiting before other symptoms, says gastroenterologist Michael Kattah, M.D., Ph.D., a UCSF assistant professor. If you swallow virus particles, he says, there's a good chance they will infect cells lining your stomach, small intestine, or colon. As in the lungs and heart, these cells are studded with vulnerable ACE2 portals.
Especially disconcerting, Kattah says, is how long the virus seems to persist in the gut. About 50 percent of patients with COVID-19 have virus particles in their stools, often for weeks after their nose swabs test negative, he points out. Laboratory studies show that these particles are often still alive and can infect cells in a petri dish. Whether fecal transmission occurs between people, however, is an open question. If the answer is yes, people recovering from COVID-19 may need to stay quarantined even after they feel well, and the rest of us will need to be as meticulous about bathroom hygiene as we've become about handwashing and mask-wearing.
Other specialists are also raising flags. Neurologists worry about reports of COVID-19 patients with headaches, "brain fog," loss of the sense of smell, dizziness, delirium, and, in rare cases, stroke. Nephrologists worry about kidney stress and failure. Hepatologists worry about liver injuries. Ophthalmologists worry about pink eye. Pediatricians, meanwhile, worry about a peculiar COVID-related inflammatory syndrome that's showing up in kids and young adults.
Researchers are still sorting out the causes for this constellation of effects. If you come down with a particular symptom, is it because the virus is attacking your cells? Because your immune system is overreacting? Or just because you're very sick? In any severe illness, for example, the kidneys must work extra hard to filter waste and control nutrients and fluid; if overtaxed, they may begin to fail. Similarly, cognitive problems can result from increased blood toxins due to stressed kidneys or from low oxygen due to respiratory distress. "There's a lot of smoke," says Michael Wilson, M.D. '07, MAS '16, the Rachleff Distinguished Professor at UCSF's Weill Institute for Neurosciences. "We need to figure out where the fire is coming from."
Recently, there's been speculation that some of COVID-19's seemingly disparate symptoms may stem from trouble in the blood. Blood clots, for example, are showing up in cases of COVID-19 frequently enough for clinicians to take notice. "There's something unique about the coagulation system in these patients," says nephrologist Kathleen Liu, M.D. '99, Ph.D. '97, MAS '07, a UCSF professor of medicine. In caring for COVID-19 patients on dialysis machines, she's been surprised to see blood clots block dialysis tubes more than usual. Clotted tubes are common, she says, "but this is extreme."
That may be because, as growing evidence suggests, SARS-CoV-2 can infect cells in the walls of blood vessels that help regulate blood flow and coagulation, or clotting. If true, this behavior could explain some of the virus's weirder (and rarer) manifestations, such as heart attacks, strokes, and even "COVID toes."
 Most symptomatic cases of COVID-19 are mild. Credit: UCSF
Most symptomatic cases of COVID-19 are mild. Credit: UCSF"Our vasculature is a contiguous system," says cardiologist Parikh. "Thus injury in one area, such as blood vessels in the lungs, can set off clotting cascades that affect multiple organs." Some of that trouble likely results from inflammation triggered by the immune system, she points out, although another culprit may be the body's RAAS, or renin-angiotensin-aldosterone system, a hormone system that controls blood pressure and fluid balance. Because RAAS involves ACE2 receptors, Parikh suspects it may become disrupted when the virus infects cells through these receptors, thus triggering coagulation and other downstream effects. Her lab is now studying this system in COVID-19 patients to better understand how SARS-CoV-2 infection affects it.
Inevitably, some ailments may turn out to be red herrings. During a pandemic, when people are flocking to hospitals with infections, clinicians will also see a rise in other health problems, simply by the rules of statistics, points out S. Andrew Josephson, M.D., the Francheschi-Mitchell Professor, chair of UCSF's neurology department, and a member of the Weill Institute for Neurosciences. "If the prevalence of infection is high, then almost any condition—a broken leg, if you will—you might conclude is associated with COVID-19."
"As clinicians, we want to get information to our medical community and to the public as quickly as possible," Josephson says, "but we have to be cautious about not making too big a deal of a little blip."
The long tail
As with any infection, how long a bout of COVID-19 lasts varies from person to person. If you're ill enough to need critical care, you can expect the disease to take at least a few weeks to run its course. In some cases, symptoms persist for months. For a typical milder case, though, you should feel better within a couple weeks.
At that point, the question foremost on your mind will be: Am I immune? There are now more than a dozen antibody tests on the market, but most are unreliable, according to UCSF research. And even the best tests can't tell you whether you have enough of the right kinds of antibodies to protect you against reinfection. "There is a lot of hope and belief that we'll have an antibody test that actually informs us of immunity, but we're not quite there yet," says Chaz Langelier, M.D., Ph.D., a UCSF assistant professor of medicine who is working to improve diagnostic tools for COVID-19.
What we have in the meantime are a lot of unknowns: If you do become immune to SARS-CoV-2, when and how does that occur? Will you gain immunity from a mild or asymptomatic case, as well as a severe one? How long will that immunity last?
"The answers will have huge implications for social distancing and masking and for getting the economy back up and running," says Michael Peluso, M.D., a clinical fellow who came to UCSF three years ago to help fight HIV. Now he's co-leading a new study called LIINC (Long-term Impact of Infection with Novel Coronavirus), which is enrolling people who have been infected with SARS-CoV-2 and will follow them for two years. Besides illuminating changes in immunity over time, LIINC is investigating chronic effects of infection on the immune system, lungs, heart, brain, blood, and other parts of the body.
"I hope people will recover and immunity will be protective and long-lasting, and that will be that," Peluso says.
It's what we all hope. We hope we will beat an infection swiftly—or, better yet, avoid the virus until there is a vaccine. We hope that if we do fall gravely ill, we will be cared for by the best providers and tended to by people we love. The reality, as we already know, is more complicated. And even if COVID-19 doesn't batter our bodies, the pandemic will surely leave scars—on our psyches, our livelihoods, our institutions, and our health—that we are only beginning to fathom. In truth, we don't know how our cards will fall, as individuals or as a people. Only time—and data—will tell.
Provided by University of California, San Francisco
No comments:
Post a Comment