A new study proposes a solution to a question that perplexed Darwin: How do corals thrive in barren seascapes?
BYMOLLY RAINS

After Charles Darwin happened across “vast rings of coral-rock” while voyaging through the southern Pacific Ocean on the Beagle, he wrote that upon seeing them, “everyone must be struck with astonishment.” These encounters inspired in the naturalist a lifelong fascination with coral reefs and one persistent question: How do vibrant corals flourish in often-barren ocean landscapes?
Known as the Darwin Paradox, this mystery has continued to puzzle generations of oceanographers. A new study published today in Nature offers a solution. According to its authors, corals make up for nutrient scarcity by harvesting and feeding on their resident algae, like hungry farmers. It’s “a really, really beautiful study,” says coral biologist Mónica Medina of Pennsylvania State University, who was not involved in the work.
Researchers have long known that corals maintain a mutually beneficial relationship with the single-celled algae that call the animals’ tissue home. There, sheltered from the harsh conditions of the open ocean, the algae feed on the corals’ waste products. In return, the algae convert sunlight into energy-rich food molecules that nourish themselves and their hosts. Corals also feed on drifting zooplankton to capture other essential nutrients
But these food sources alone can’t account for the world’s vast and prolific coral reefs. “There are lots of studies that have examined [coral] nutrient transport and tried to do the math, but it never really quite made perfect sense,” says Virginia Weis, a marine physiologist at Oregon State University who was not involved in the study. In particular, the useful forms of nutrients that corals need in order to grow—including nitrogen and phosphorus—tend to be in short supply around reefs.
However, sea creatures living in the vicinity excrete plenty of inorganic nitrogen and phosphorus, which the coral’s algal residents can readily consume. Jörg Wiedenmann, head of the coral reef laboratory at the University of Southampton, wondered whether there was a connection. Could the algae somehow be passing these nutrients along to their coral hosts?
To find out, Wiedenmann and colleagues placed coral samples in tanks without any food. To half of the tanks, they added inorganic nutrients that only the coral’s resident algae could enjoy. Corals in the tanks containing no nutrients at all stopped growing and had lost about half of their algal partners after just 50 days, giving them a bleached and stunted appearance. But in the tanks where the algae had been fed, the coral grew robustly. Scientists knew algae provided corals with energy via photosynthesis, but these results suggested they were also sustaining coral growth by converting inorganic nitrogen and phosphorus into coral chow. Yet how the algae transferred those nutrients remained a mystery.
Wiedenmann wondered whether the corals were eating their algal partners. The team calculated the expected growth of the coral’s algal population given the input of nutrients, then compared its prediction with the actual number of algal cells in the tanks. They found far fewer algae than expected. What’s more, the amount of nitrogen and phosphorus contained within the unaccounted-for cells aligned with the nutrients required for the coral to grow as big as it did. Wiedenmann was convinced: The corals were preying on the algae.
Looking for evidence of this phenomenon in nature, the team observed the reef growth in the Indian Ocean’s Chagos archipelago. Here, seabirds live in high density on some islands but not others, and the birds’ droppings contain inorganic nutrients that algae can eat directly, but that coral cannot. Over a period of 3 years, the researchers found that reefs near islands with lots of birds—and, therefore, plenty of algae food—grew twice as fast as those near islands where birds were scarce. A unique form of nitrogen that is plentiful in bird droppings, but not zooplankton, also reappeared in corals around bird-dense islands. To the researchers, this was further evidence that the algae were indeed shuttling nutrients from the birds to their coral hosts.
The study nicely combines laboratory and fieldwork to crack a long-standing mystery, Medina says. It could also help scientists better understand the devastating effects of coral bleaching, she adds, in which the relationship between corals and their algal residents breaks down.
- Article
- Open Access
- Published:
Reef-building corals farm and feed on their photosynthetic symbionts
Nature (2023)
Abstract
Coral reefs are highly diverse ecosystems that thrive in nutrient-poor waters, a phenomenon frequently referred to as the Darwin paradox1. The energy demand of coral animal hosts can often be fully met by the excess production of carbon-rich photosynthates by their algal symbionts2,3. However, the understanding of mechanisms that enable corals to acquire the vital nutrients nitrogen and phosphorus from their symbionts is incomplete4,5,6,7,8,9. Here we show, through a series of long-term experiments, that the uptake of dissolved inorganic nitrogen and phosphorus by the symbionts alone is sufficient to sustain rapid coral growth. Next, considering the nitrogen and phosphorus budgets of host and symbionts, we identify that these nutrients are gathered through symbiont ‘farming’ and are translocated to the host by digestion of excess symbiont cells. Finally, we use a large-scale natural experiment in which seabirds fertilize some reefs but not others, to show that the efficient utilization of dissolved inorganic nutrients by symbiotic corals established in our laboratory experiments has the potential to enhance coral growth in the wild at the ecosystem level. Feeding on symbionts enables coral animals to tap into an important nutrient pool and helps to explain the evolutionary and ecological success of symbiotic corals in nutrient-limited waters.
CONTINUE READING:
Reef-building corals farm and feed on their photosynthetic symbionts | Nature
Darwin’s Paradox of Coral Reefs Solved – Scientists Unravel Age-Old Mystery

A research study from the University of Southampton has unveiled that corals feed on microscopic algae living within their cells, accessing a nutrient source previously thought unavailable. This discovery answers a long-standing mystery known as Darwin’s Paradox of Coral Reefs, explaining how corals flourish in nutrient-poor waters.
A new study led by the University of Southampton in the UK has uncovered why coral reefs flourish in waters that appear to be deficient in nutrients, a phenomenon that has fascinated scientists since Charles Darwin.
The research shows that corals farm and feed on their photosynthetic symbionts – microscopic algae that live inside their cells. This vegetarian diet allows the corals to tap into a large pool of nutrients that was previously considered unavailable to them. Effectively, they are eating some of their symbiont algae to get the nutrition they need to survive.
Professor Jörg Wiedenmann, Head of the Coral Reef Laboratory at the University of Southampton, who led the study comments: “The question as to why coral reefs thrive in parts of the oceans that are poor in nutrients is known as Darwin’s Paradox of Coral Reefs and has inspired the discovery of several important processes that can help to explain this phenomenon. We can now add the missing piece of the puzzle and help to solve the long-running mystery.”

Reef corals provide home and feeding grounds for many organisms. Credit: Wiedenmann / D’Angelo / University of Southampton
He continues: “When Charles Darwin set sail on the HMS Beagle, he considered himself a geologist and during his voyage through tropical seas, quickly became interested in where and why coral reefs are formed. Darwin correctly predicted how the subsidence of the Earth’s crust and the steady upward growth of corals interact to form vast reef structures. However, the biological mechanisms behind this vigorous growth remained unstudied.”
Surviving together
Stony corals are soft-bodied creatures that may look like plants to some, but are in fact animals. These organisms are made up of many individual polyps that live together as a colony and secret limestone skeletons which form the three-dimensional framework we know as ‘reefs’.
Coral reefs are important underwater ecosystems that benefit many human communities. They provide a home and feeding ground for countless organisms, sustaining about 25 percent of global ocean biodiversity. Thereby, they deliver food and income to about half a billion people on Earth.
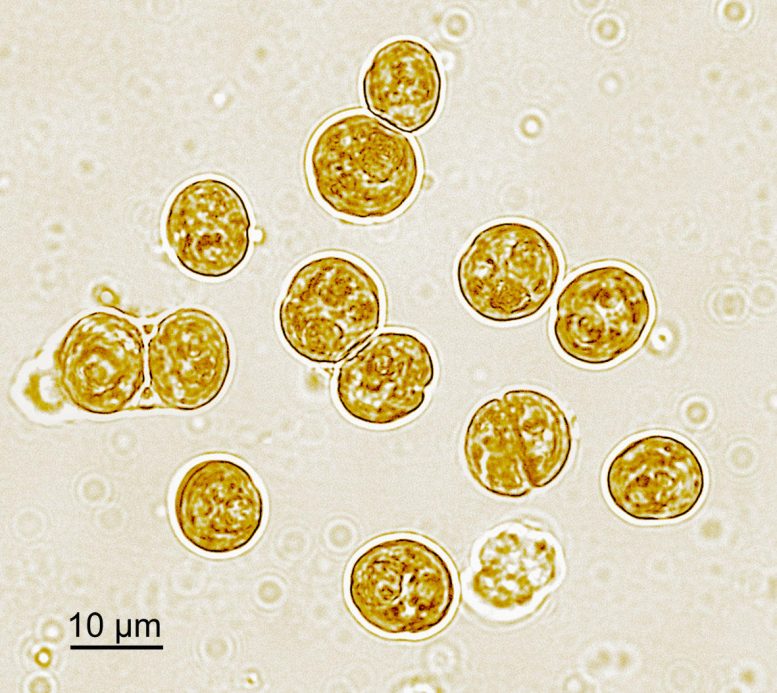
Unicellular symbiont algae of a reef coral showing growth by cell division. Credit: Wiedenmann / D’Angelo / University of Southampton
The coral animals are dependent on a ‘symbiosis’, a mutually beneficial relationship with microscopic algae that live inside their cells. The photosynthetic algae produce large amounts of carbon-rich compounds, such as sugars, which they transfer to the host coral for energy generation.
The symbiont algae are also very efficient in taking up dissolved inorganic nutrients from seawater, such as nitrate and phosphate. Even in nutrient-poor oceans, these compounds can be found in considerable amounts as excretion products of organisms, such as sponges, that live close by. They can also be transferred to reefs by ocean currents.
What the scientists found
In contrast to their symbionts, the coral host cannot absorb or use dissolved inorganic nutrients directly and, until now, it was unclear how these nutrients could fuel the growth of coral. However, the mechanism by which these essential growth nutrients are transferred to the coral animals has been identified by scientists from the University of Southampton, working with a team of collaborators including Lancaster University in the UK, Tel Aviv University, and the University of Jerusalem in Israel.
Their findings are published in the journal Nature.

Experimental aquarium of the Coral Reef Laboratory at the University of Southampton. Credit: Wiedenmann / D’Angelo / University of Southampton
By performing a series of long-term experiments at the University of Southampton’s Coral Reef Laboratory, the scientists demonstrated that corals actually digest some of their symbiont population to access the nitrogen and phosphorus that symbionts absorb from the water. Where there are sufficient dissolved inorganic nutrients in the water, this mechanism allows corals to grow quickly, even if they do not receive any additional food. Results from fieldwork in remote coral reef atolls in the Indian Ocean support the lab findings, demonstrating that this mechanism boosts coral growth in the wild at the ecosystem level.
Dr Cecilia D’Angelo, Associate Professor of Coral Biology at Southampton and one of the lead authors, comments: “Over the many years during which we propagated symbiotic corals in our experimental aquarium system, we had observed that they grew very well even when they were not fed. It could not be explained by the current state of knowledge how nutrients were exchanged by the two partners of the symbiosis, so we figured that we were missing a big piece of the picture and started to analyze the process systematically.”

Seabirds introduce nutrients in coral reefs in the Indian Ocean. Credit: Nick Graham, Lancaster University
Dr Loreto Mardones-Velozo, a researcher in the Coral Reef Laboratory who conducted key experiments, adds: “One would expect that animals die or stop growing if they don’t eat. However, the corals looked perfectly happy and grew rapidly if we kept them in water with elevated levels of dissolved inorganic nutrients.”
The science behind the findings
The researchers used a specifically labeled chemical compound to track the movement of the essential nutrient nitrogen between the partners of the symbiosis. Nitrogen in the chemical form used in the experiments can be only integrated in their cells by the symbionts, but not the coral host.
Bastian Hambach, Manager of the Stable Isotope Mass Spectrometry Laboratory at the University of Southampton, explains: “We used isotopic labeling to ‘spike’ the nutrients supplied to the corals with nitrogen atoms that were heavier than normal. These isotopes allowed us to trace the coral’s use of the nutrients using ultrasensitive detection methods.”

Dr. Cecilia D’Angelo propagating corals in the Coral Reef Laboratory at the University of Southampton. Credit: Wiedenmann / D’Angelo / University of Southampton
Professor Paul Wilson, paleoceanographer at the University of Southampton expands: “With this technique, we could unambiguously demonstrate that the nitrogen atoms that sustained the growth of the coral tissue were derived from the dissolved inorganic nutrients that were fed to their symbionts in the experiment.”
Professor Jörg Wiedenmann of the University of Southampton adds: “We used 10 different coral species to quantify how the symbiont population grew along with their hosts. Using mathematical models of the symbiont growth, we could show that the corals digest the excess part of their symbiont population to harvest nutrients for their growth. Our data suggest that most symbiotic corals can supplement their nutrition through such a ‘vegetarian diet’.”
The scientists also analyzed corals growing around islands in the Indian Ocean, some with seabirds on them and some without, to show that corals have the potential to farm and feed on their symbionts in the wild.
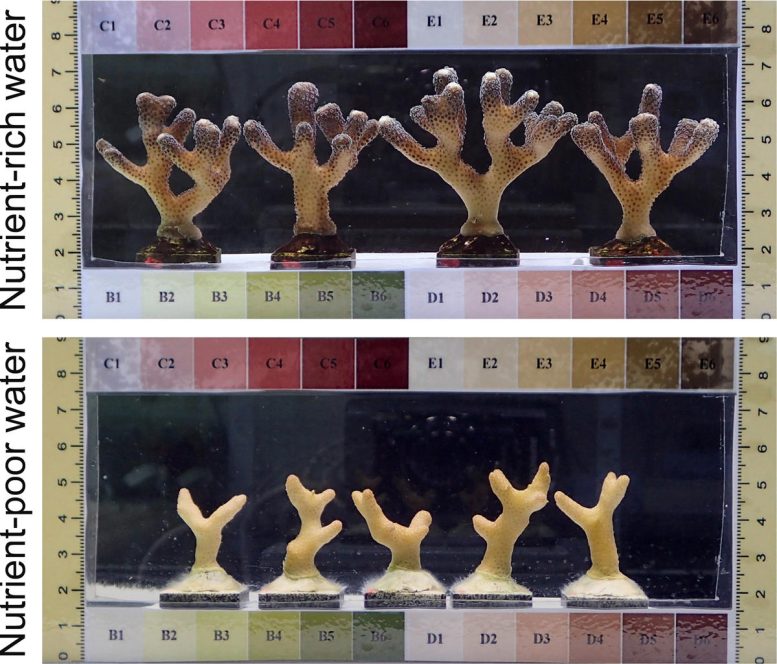
Growth of the experimental coral Stylophora pistillata. Credit: Mardones-Velozo / D’Angelo / Wiedenmann / University of Southampton
Professor Nick Graham, Marine Ecologist from Lancaster University, explains: “The reefs around some of these islands are supplied with substantial amounts of nutrients that come from ‘guano’, the excrements of the seabirds nesting on the islands. On other islands, the seabird colonies have been decimated by invasive rats. Accordingly, the associated reefs receive less nutrients. We measured the growth of staghorn coral colonies around islands with and without dense seabird populations and found that growth was more than twice as fast on reefs that were supplied with seabird nutrients.
“We calculate that about half of the nitrogen molecules in the tissue of the coral animals from islands with seabirds can be traced back to uptake by the symbionts and the subsequent translocation to the host.”

Scientist monitoring coral growth on Indian Ocean Reefs to study the effect of seabird nutrients. Credit: Nick Graham, Lancaster University
Global warming and the future
Excessive nutrient enrichment, often caused by human activities, can damage corals and represents a growing threat in many reefs. However, some coral reefs might receive less nutrients in the future as global warming may cut them off from some of their natural supply routes.
Dr D’Angelo from the University of Southampton explains: “Warming surface waters are less likely to receive nutrients from deeper water layers. The reduced water productivity can result in less nutrients for the symbionts and in turn less food for the coral animals.
The scientists’ new findings suggest that while coral animals may endure brief periods of starvation by feeding off their symbionts, some coral reefs might be at risk of starvation in response to more prolonged nutrient depletion brought on by global warming in some areas.
Reference: “Reef-building corals farm and feed on their photosynthetic symbionts” by Jörg Wiedenmann, Cecilia D’Angelo, M. Loreto Mardones, Shona Moore, Cassandra E. Benkwitt, Nicholas A. J. Graham, Bastian Hambach, Paul A. Wilson, James Vanstone, Gal Eyal, Or Ben-Zvi, Yossi Loya and Amatzia Genin,
23 August 2023, Nature.
DOI: 10.1038/s41586-023-06442-5
No comments:
Post a Comment