China's control measures may have prevented 700,000 COVID-19 cases: study
by Pennsylvania State University
Credit: CC0 Public Domain
China's control measures during the first 50 days of the COVID-19 epidemic may have delayed the spread of the virus to cities outside of Wuhan by several days and, by interrupting transmission nationwide, prevented more than 700,000 infections across the country, according to an international team of researchers. The findings, published today (March 31) in the journal Science, could be useful to countries that are still in early phases of the COVID-19 outbreak.
"The number of confirmed cases in China by day 50 (February 19) of the epidemic, was around 30,000," said Christopher Dye, visiting professor of zoology and visiting fellow at the Oxford Martin School, University of Oxford. "Our analysis suggests that without the Wuhan travel ban and the national emergency response there would have been more than 700,000 confirmed COVID-19 cases outside of Wuhan by that date. China's control measures appear to have worked by successfully breaking the chain of transmission—preventing contact between infectious and susceptible people."
The researchers used a unique combination of case reports, human movement data and public health intervention information to investigate the spread and control of COVID-19. They examined the movements of 4.3 million people out of Wuhan before the travel ban, the types and timing of control measures implemented across the cities of China and the numbers of COVID-19 cases reported each day in every city.
"One fascinating aspect of our work is that it shows the power of novel data streams such as cell phone mobility data," said Ottar Bjornstad, distinguished professor of entomology and biology, Penn State. "Since the time period we studied included the Spring Festival holiday and Chinese Lunar New Year, we were able to compare patterns of travel into and out of Wuhan during the outbreak with cell phone data from two previous spring festivals. The analysis revealed an extraordinary reduction in movement following the travel ban of January 23, 2020. Based on this data, we could also calculate the likely reduction in Wuhan-associated cases in other cities across China."
The team's model also analyzed the specific effects of the Wuhan shutdown and found that it delayed the arrival of COVID-19 in other cities by several days. "This delay provided extra time to prepare for the arrival of COVID-19 in more than 130 cities," said Huaiyu Tian, associate professor of epidemiology, Beijing Normal University.
These cities banned public gatherings, closed entertainment venues and suspended public transport, among other actions. As a result, they reported 33% fewer confirmed cases during the first week of their outbreaks than cities that did not implement a Level 1 Response.
While the control measures taken thus far have reduced the number of COVID-19 infections to very low levels, China, is by no means out of the woods.
"Given the small fraction of the Chinese population that has been infected, a much larger number of people remains at risk of COVID-19," said Tian. "We are acutely aware that resident or imported infections could lead to a resurgence of transmission."
Bjornstad noted that SARS-CoV-2 may establish as a human endemic globally in the years to come.
"It is critical to keep in mind that this virgin epidemic likely will affect people of different ages and susceptibilities, and therefore have different fatality levels, than possible subsequent seasonal epidemics," he said.
Travel restrictions are most useful in the early and late phase of an epidemic: study
More information: Science (2020). DOI: 10.1126/science.abb6105
Coronavirus measures may have already averted up to 120,000 deaths across Europe
by Ryan O'hare, Dr Sabine L. Van Elsland, Imperial College London
Credit: Imperial College London
Strong social distancing measures to slow and suppress the spread of COVID-19 across Europe are estimated to have averted thousands of deaths.
The findings come from a new analysis by researchers at Imperial College London, which estimates the potential impact of interventions in 11 European countries to counter the coronavirus pandemic—including school closures and national lockdowns.
According to the research, up to 120,000 deaths may have already been averted in 11 countries, including the UK, Italy and Spain. However, they add that the estimated proportion of people to have been infected with the virus may only be between 2 to 12% of the population (2.7% in the UK).
The report is the thirteenth to be released by The WHO Collaborating Centre for Infectious Disease Modelling within the MRC Centre for Global Infectious Disease Analysis (GIDA), Abdul Latif Jameel Institute for Disease and Emergency Analytics (J-IDEA).
Europe-wide response
Many European countries have now implemented unprecedented measures to mitigate the impact of COVID-19, including isolation of confirmed and suspected cases, closing schools and universities, banning mass-gatherings, and most recently, wide-scale social distancing including local and national lockdowns.
Such interventions are aimed at managing the epidemic to prevent an unmitigated rise in cases which would overload health care capacity. Now, the latest modeling shows that they may be having a significant impact, potentially averting up to 120,000 deaths across Europe.
Dr. Samir Bhatt, report author and Senior Lecturer from the School of Public Health, said: "It is of course a difficult time for Europe, but governments have taken significant steps to ensure health systems do not get overwhelmed. There is sound evidence that these have started to work and have flattened the curve.
"We believe a large number of lives have been saved. However, it is too soon to say if we have managed to fully control epidemics and more difficult decisions will need to be taken in the coming weeks"
Dr. Seth Flaxman, first author on the latest study, added: "Even as the death toll continues to mount, we see enough signal in the data to conclude that sustained, drastic actions taken by European governments have already saved lives by driving down the number of new infections each day.
"But because these interventions are very recent in most countries, and there is a lag between infection and death, it will take longer—from days to weeks—for these effects to be reflected in the number of daily deaths."
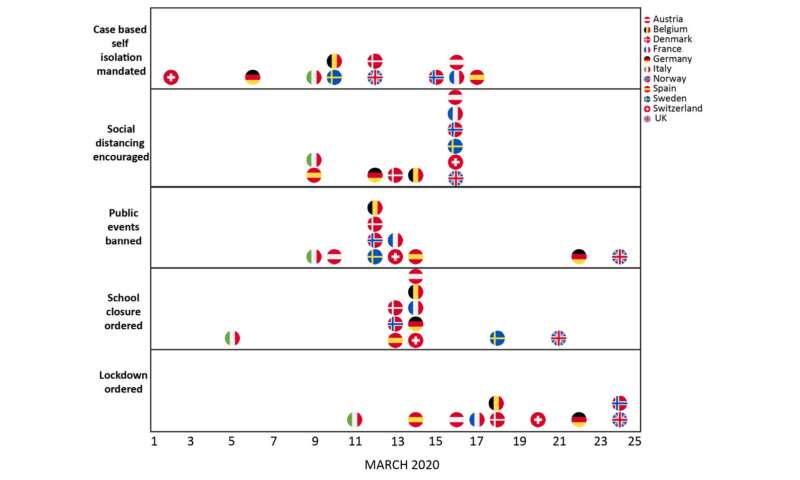
Intervention timings for the 11 European countries included in the analysis. Credit: Imperial College London
Modeling the impact
In the latest report, researchers aimed to model the likely impact of interventions in place on reducing loss of life. The team used real-time daily data from the European Centre of Disease Control (ECDC) on the number of deaths in 11 European countries: Austria, Belgium, Denmark, France, Germany, Italy, Norway, Spain, Sweden, Switzerland and the United Kingdom.
The models focused on reproductive number—the average number of new infections generated by each infected person. It was assumed that changes in reproductive number are an immediate response to these interventions being implemented, rather than broader gradual changes in behavior. Overall, the models estimate that countries have managed to reduce their reproductive number.
The team's analysis shows that with the current interventions remaining in place, that measures across all 11 countries will have averted between 21,000 and 120,000 deaths up to 31 March. They add that many more deaths will be averted by keeping interventions in place until transmission drops to low levels.
"Our results suggest that interventions such as social distancing or lockdowns have already saved many lives and will continue to save lives," explained Professor Axel Gandy, Chair of Statistics within the Department of Mathematics. "The impact of the pandemic is extreme—but it would have been much worse without the interventions. Keeping interventions in place is crucial for controlling it."
In addition to reducing deaths, the latest report estimates that between 7 and 43 million people have been infected with the coronavirus (SARS-CoV-2) across all 11 countries up to 28th March, representing between 1.88% and 11.43% of the population.
Given the lag of 2-3 weeks between when transmission changes occur and when their impact can be observed in trends in deaths, it may still be too early to show for most of the 11 countries that recent interventions have been effective.
The researchers stress that the results are strongly driven by the data from countries with more advanced epidemics, and earlier interventions. It is critical, they explain, that the current social distancing measures remain in place, and trends in cases and deaths are closely monitored in the coming days and weeks to provide reassurance that transmission of the virus is slowing.
Professor Christl Donnelly, Professor of Statistical Epidemiology within the School of Public Health, said: "Europeans, like many people elsewhere, have changed their lives profoundly in recent weeks. This report makes clear early evidence of the benefits of these social distancing measures. By keeping our distance from each other, we limit the opportunities for the virus to spread and reduce the risks of illness and even death among those around us."
Professor Neil Ferguson, Director of J-IDEA at Imperial, added: "This analysis show that the interventions European countries have put in place have significantly slowed the spread of COVID-19. However, it is not yet clear whether or how quickly these measures will cause the numbers of new cases to decline. Data collected in the next two weeks will be crucial to refining our assessment of this key point."
Report author Dr. Swapnil Mishra, a Research Associate within the School of Public Health, said: "We implement a novel scientific model of the epidemic within a robust statistical framework. It is a fully Bayesian analysis, so we do not just look at a single scenario, but rather thousands of plausible scenarios and counterfactuals. Our analysis suggests in these difficult times interventions are required and necessary to keep the pandemic in control."
Explore furtherFollow the latest news on the coronavirus (COVID-19) outbreak